![[drawing of line spectra of argon, zinc and hydrogen]](RAYLEIGH2.jpg)
It is appropriate to consider the discovery of argon among papers about the periodic system, because the atomic weight of argon and its properties were such that there seemed no place for it in the periodic system of the time [Giunta 2001]. We will see the placement of argon and its family of elements proposed in the next chapter. Meanwhile Rayleigh's lecture provides a compelling example of careful scientific practice, tracking down a small anomaly to what proved to be an interesting and important discovery [Giunta 1998].
It is some three or four years since I had the honour of lecturing here one Friday evening upon the densities of oxygen and hydrogen gases, and upon the conclusions that might be drawn from the results. It is not necessary, therefore, that I should trouble you to-night with any detail as to the method by which gases can be accurately weighed. I must take that as known, merely mentioning that it is substantially the same as is used by all investigators nowadays, and introduced more than fifty years ago by Regnault. It was not until after that lecture that I turned my attention to nitrogen[2]; and in the first instance I employed a method of preparing the gas which originated with Mr Vernon Harcourt, of Oxford. In this method the oxygen of ordinary atmospheric air is got rid of with the aid of ammonia. Air is bubbled through liquid ammonia, and then passed through a red-hot tube. In its passage the oxygen of the air combines with the hydrogen of the ammonia, all the oxygen being in that way burnt up and converted into water. The excess of ammonia is subsequently absorbed with acid, and the water by ordinary desiccating agents.[3] That method is very convenient; and, when I had obtained a few concordant results by means of it, I thought that the work was complete, and that the weight of nitrogen was satisfactorily determined.[4] But then I reflected that it is always advisable to employ more than one method, and that the method I had used--Mr Vernon Harcourt's method--was not that which had been used by any of those who had preceded me in weighing nitrogen.[5] The usual method consists in absorbing the oxygen of air by means of red-hot copper; and I thought that I ought at least to give that method a trial, fully expecting to obtain forthwith a value in harmony with that already afforded by the ammonia method.[6] The result, however, proved otherwise. The gas obtained by the copper method, as I may call it, proved to be one-thousandth part heavier than that obtained by the ammonia method; and, on repetition, that difference was only brought out more clearly.[7] This was about three years ago. In order, if possible, to get further light upon a discrepancy which puzzled me very much[8], and which, at that time, I regarded only with disgust and impatience, I published a letter in Nature inviting criticisms from chemists who might be interested in such questions.[9] I obtained various useful suggestions, but none going to the root of the matter. Several persons who wrote to me privately were inclined to think that the explanation was to be sought in a partial dissociation of the nitrogen derived from ammonia. For, before going further, I ought to explain that, in the nitrogen obtained by the ammonia method, some--about a seventh part--is derived from the ammonia, the larger part, however, being derived as usual from the atmosphere.[10] If the chemically derived nitrogen were partly dissociated into its component atoms, then the lightness of the gas so prepared would be explained.[11]
The next step in the enquiry was, if possible, to exaggerate the discrepancy. One's instinct at first is to try to get rid of a discrepancy, but I believe that experience shows such an endeavour to be a mistake. What one ought to do is to magnify a small discrepancy with a view to finding out the explanation; and, as it appeared in the present case that the root of the discrepancy lay in the fact that part of the nitrogen prepared by the ammonia method was nitrogen out of ammonia, although the greater part remained of common origin in both cases, the application of the principle suggested a trial of the weight of nitrogen obtained wholly from ammonia.[12] This could easily be done by substituting pure oxygen for atmospheric air in the ammonia method, so that the whole, instead of only a part, of the nitrogen collected should be derived from the ammonia itself. The discrepancy was at once magnified some five times. The nitrogen so obtained from ammonia proved to be about one-half per cent. lighter than nitrogen obtained in the ordinary way from the atmosphere, and which I may call for brevity "atmospheric" nitrogen.
That result stood out pretty sharply from the first; but it was necessary to confirm it by comparison with nitrogen chemically derived in other ways. The table before you gives a summary of such results, the numbers being the weights in grams actually contained under standard conditions in the globe employed.[13]
ATMOSPHERIC NITROGEN. By hot copper (1892) 2.3103 By hot iron (1893) 2.3100 By ferrous hydrate (1894) 2.3102 mean 2.3102
CHEMICAL NITROGEN. From nitric oxide 2.3001 From nitrous oxide 2.2990 From ammonium nitrite purified at a red heat 2.2987 From urea 2.2985 From ammonium nitrite purified in the cold 2.2987 mean 2.2990
The difference is about 11 milligrams, or about one-half per cent.; and it was sufficient to prove conclusively that the two kinds of nitrogen--the chemically derived nitrogen and the atmospheric nitrogen--differed in weight, and therefore, of course, in quality, for some reason hitherto unknown.[14]
I need not spend time in explaining the various precautions that were necessary in order to establish surely that conclusion. One had to be on one's guard against impurities, especially against the presence of hydrogen, which might seriously lighten any gas in which it was contained.[15] I believe, however, that the precautions taken were sufficient to exclude all questions of that sort, and the result, which I published about this time last year, stood sharply out, that the nitrogen obtained from chemical sources was different from the nitrogen obtained from the air.
Well, that difference, admitting it to be established, was sufficient to show that some hitherto unknown gas is involved in the matter. It might be that the new gas was dissociated nitrogen, contained in that which was too light, the chemical nitrogen--and at first that was the explanation to which I leaned; but certain experiments went a long way to discourage such a supposition. In the first place, chemical evidence--and in this matter I am greatly dependent upon the kindness of chemical friends--tends to show that, even if ordinary nitrogen could be dissociated at all into its component atoms, such atoms would not be likely to enjoy any very long continued existence.[16] Even ozone goes slowly back to the more normal state of oxygen; and it was thought that dissociated nitrogen would have even a greater tendency to revert to the normal condition. The experiment suggested by that remark was as follows--to keep chemical nitrogen--the too light nitrogen which might be supposed to contain dissociated molecules--for a good while, and to examine whether it changed in density. Of course it would be useless to shut up gas in a globe and weigh it, and then, after an interval, to weigh it again, for there would be no opportunity for any change of weight to occur, even although the gas within the globe had undergone some chemical alteration. It is necessary to re-establish the standard conditions of temperature and pressure which are always understood when we speak of filling a globe with gas, for I need hardly say that filling a globe with gas is but a figure of speech. Everything depends upon the temperature and pressure at which you work. However, that obvious point being borne in mind, it was proved by experiment that the gas did not change in weight by standing for eight months--a result tending to show that the abnormal lightness was not the consequence of dissociation.[17]
Further experiments were tried upon the action of the silent electric discharge--both upon the atmospheric nitrogen and upon the chemically derived nitrogen--but neither of them seemed to be sensibly affected by such treatment; so that, altogether, the balance of evidence seemed to incline against the hypothesis of abnormal lightness in the chemically derived nitrogen being due to dissociation, and to suggest strongly, as almost the only possible alternative, that there must be in the atmospheric nitrogen some constituent heavier than true nitrogen.[18]
At that point the question arose, What was the evidence that all the so-called nitrogen of the atmosphere was of one quality? And I remember--I think it was about this time last year, or a little earlier--putting the question to my colleague, Professor Dewar. His answer was that he doubted whether anything material had been done upon the matter since the time of Cavendish, and that I had better refer to Cavendish's original paper.[19] That advice I quickly followed, and I was rather surprised to find that Cavendish had himself put this question quite as sharply as I could put it. Translated from the old-fashioned phraseology connected with the theory of phlogiston, his question was whether the inert ingredient of the air is really all of one kind; whether all the nitrogen of the air is really the same as the nitrogen of nitre.[20] Cavendish not only asked himself this question, but he endeavoured to answer it by an appeal to experiment.
I should like to show you Cavendish's experiment in something like its original form. He inverted a U-tube filled with mercury, the legs standing in two separate mercury cups. He then passed up, so as to stand above the mercury, a mixture of nitrogen, or of air, and oxygen; and he caused an electric current from a frictional electrical machine like the one I have before me to pass from the mercury in the one leg to the mercury in the other, giving sparks across the intervening column of air.[21] I do not propose to use a frictional machine to-night, but I will substitute for it one giving electricity of the same quality of the construction introduced by Mr Wimshurst, of which we have a fine specimen in the Institution. It stands just outside the door of the theatre, and will supply an electric current along insulated wires, leading to the mercury cups; and, if we are successful, we shall cause sparks to pass through the small length of air included above the columns of mercury. There they are; and after a little time you will notice that the mercury rises, indicating that the gas is sensibly absorbed under the influence of the sparks and of a piece of potash floating on the mercury. It was by that means that Cavendish established his great discovery of the nature of the inert ingredient in the atmosphere, which we now call nitrogen;[22] and, as I have said, Cavendish himself proposed the question, as distinctly as we can do, Is this inert ingredient all of one kind? and he proceeded to test that question. He found, after days and weeks of protracted experiment, that, for the most part, the nitrogen of the atmosphere was absorbed in this manner, and converted into nitrous acid; but that there was a small residue remaining after prolonged treatment with sparks, and a final absorption of the residual oxygen.[23] That residue amounted to about 1/120 part of the nitrogen taken; and Cavendish draws the conclusion that, if there be more than one inert ingredient in the atmosphere, at any rate the second ingredient is not contained to a greater extent than 1/120 part.
I must not wait too long over the experiment. Mr Gordon tells me that a certain amount of contraction has already occurred; and if we project the U upon the screen, we shall be able to verify the fact. It is only a question of time for the greater part of the gas to be taken up, as we have proved by preliminary experiments.
In what I have to say from this point onwards, I must be understood as speaking as much on behalf of Professor Ramsay as for myself. At the first, the work which we did was to a certain extent independent. Afterwards we worked in concert, and all that we have published in our joint names must be regarded as being equally the work of both of us. But, of course, Professor Ramsay must not be held responsible for any chemical blunder into which I may stumble to-night.[24]
By his work and by mine the heavier ingredient in atmospheric nitrogen which was the origin of the discrepancy in the densities has been isolated, and we have given it the name of "argon." For this purpose we may use the original method of Cavendish, with the advantage of modern appliances.[25] We can procure more powerful electric sparks than any which Cavendish could command by the use of the ordinary Ruhmkorff coil stimulated by a battery of Grove cells[26]; and it is possible so to obtain evidence of the existence of argon. The oxidation of nitrogen by that method goes on pretty quickly. If you put some ordinary air, or better still, a mixture of air and oxygen, in a tube in which electric sparks are made to pass for a certain time, then in looking through the tube, you observe the well-known reddish-orange fumes of the oxides of nitrogen. I will not take up time in going through the experiment, but will merely exhibit a tube already prepared (image on screen).
One can work more efficiently by employing the alternate currents from dynamo machines which are now at our command. In this Institution we have the advantage of a public supply; and if I pass alternate currents originating in Deptford through this Ruhmkorff coil, which acts as what is now called a "high potential transformer," and allow sparks from the secondary to pass in an inverted test tube between platinum points, we shall be able to show in a comparatively short time a pretty rapid absorption of the gases. The electric current is led into the working chamber through bent glass tubes containing mercury, and provided at their inner extremities with platinum points. In this arrangement we avoid the risk, which would otherwise be serious, of a fracture just when we least desired it. I now start the sparks by switching on the Ruhmkorff to the alternate current supply; and, if you will take note of the level of the liquid representing the quantity of mixed gases included, I think you will see after, perhaps, a quarter of an hour that the liquid has very appreciably risen, owing to the union of the nitrogen and the oxygen gases under the influence of the electrical discharge, and subsequent absorption of the resulting compound by the alkaline liquid with which the gas space is enclosed.[27]
By means of this little apparatus, which is very convenient for operations upon a moderate scale, such as analyses of "nitrogen" for the amount of argon that it may contain, we are able to get an absorption of about 80 cubic centimetres per hour, or about 4 inches along this test tube, when all is going well. In order, however, to effect the isolation of argon on any considerable scale by means of the oxygen method, we must employ an apparatus still more enlarged. The isolation of argon requires the removal of nitrogen, and, indeed, of very large quantities of nitrogen, for, as it appears, the proportion of argon contained in atmospheric nitrogen is only about one per cent., so that for every litre of argon that you wish to get you must eat up some hundred litres of nitrogen. That, however, can be done upon an adequate scale by calling to our aid the powerful electric discharge now obtainable by means of the alternate current supply and high potential transformers.[28]
In what I have done upon this subject I have had the advantage of the advice of Mr Crookes[29], who some years ago drew special attention to the electric discharge or flame, and showed that many of its properties depended upon the fact that it had the power of causing, upon a very considerable scale, a combination of the nitrogen and the oxygen of the air in which it was made.
I had first thought of showing in the lecture room the actual apparatus which I have employed for the concentration of argon; but the difficulty is that, as the apparatus has to be used, the working parts are almost invisible, and I came to the conclusion that it would really be more instructive as well as more convenient to show the parts isolated, a very little effort of imagination being then all that is required in order to reconstruct in the mind the actual arrangements employed.[30]
First, as to the electric arc or flame itself. We have here a transformer made by Pike and Harris. It is not the one that I have used in practice; but it is convenient for certain purposes, and it can be connected by means of a switch with the alternate currents of 100 volts furnished by the Supply Company. The platinum terminals that you see here are modelled exactly upon the plan of those which have been employed in practice. I may say a word or two on the question of mounting. The terminals require to be very massive on account of the heat evolved.[31] In this case they consist of platinum wire doubled upon itself six times. The platinums are continued by iron wires going through glass tubes, and attached at the ends to the copper leads. For better security, the tubes themselves are stopped at the lower ends with corks and charged with water, the advantage being that, when the whole arrangement is fitted by means of an indiarubber stopper into a closed vessel, you have a witness that, as long as the water remains in position, no leak can have occurred through the insulating tubes conveying the electrodes.
Now, if we switch on the current and approximate the points sufficiently, we get the electric flame.[32] There you have it. It is, at present, showing a certain amount of soda. That in time would burn off. After the arc has once been struck, the platinums can be separated; and then you have two tongues of fire ascending almost independently of one another, but meeting above. Under the influence of such a flame, the oxygen and the nitrogen of the air combine at a reasonable rate, and in this way the nitrogen is got rid of. It is now only a question of boxing up the gas in a closed space, where the argon concentrated by the combustion of the nitrogen can be collected. But there are difficulties to be encountered here. One cannot well use anything but a glass vessel. There is hardly any metal available that will withstand the action of strong alkali and of the nitrous fumes resulting from the flame. One is practically limited to glass. The glass vessel employed is a large flask with a single neck, about half full of caustic alkali. The electrodes are carried through the neck by means of an indiarubber bung provided also with tubes for leading in the gas. The electric flame is situated at a distance of only about half an inch above the caustic alkali. In that way an efficient circulation is established; the hot gases as they rise from the flame strike the top, and then as they come round again in the course of the circulation they pass sufficiently close to the caustic alkali to ensure an adequate removal of the nitrous fumes.
There is another point to be mentioned. It is necessary to keep the vessel cool; otherwise the heat would soon rise to such a point that there would be excessive generation of steam, and then the operation would come to a standstill. In order to meet this difficulty the upper part of the vessel is provided with a water-jacket, in which a circulation can be established. No doubt the glass is severely treated, but it seems to stand it in a fairly amiable manner.
By means of an arrangement of this kind, taking nearly three horse-power from the electric supply, it is possible to consume nitrogen at a reasonable rate. The transformers actually used are the "Hedgehog" transformers of Mr Swinburne, intended to transform from 100 to 2400 volts. By Mr Swinburne's advice I have used two such, the fine wires being in series so as to accumulate the electrical potential and the thick wires in parallel. The rate at which the mixed gases are absorbed is about seven litres per hour; and the apparatus, when once fairly started, works very well as a rule, going for many hours without attention. At times the arc has a trick of going out, and it then requires to be restarted by approximating the platinums. We have already worked 14 hours on end, and by the aid of one or two automatic appliances it would, I think, be possible to continue operation day and night.
The gases, air and oxygen in about equal proportions, are mixed in a large gas-holder, and are fed in automatically as required. The argon gradually accumulates; and when it is desired to stop operations the supply of nitrogen is cut off, and only pure oxygen allowed admittance. In this way the remaining nitrogen is consumed, so that, finally, the working vessel is charged with a mixture of argon and oxygen only, from which the oxygen is removed by ordinary well-known chemical methods.[33] I may mention that at the close of the operation, when the nitrogen is all gone, the arc changes its appearance and becomes of a brilliant blue colour.
I have said enough about this method, and I must now pass on to the alternative method which has been very successful in Professor Ramsay's hands--that of absorbing nitrogen by means of red-hot magnesium. By the kindness of Professor Ramsay and Mr Matthews, his assistant, we have here the full scale apparatus before us almost exactly as they use it. On the left there is a reservoir of nitrogen derived from air by the simple removal of oxygen. The gas is then dried. Here it is bubbled through sulphuric acid. It then passes through a long tube made of hard glass and charged with magnesium in the form of thin turnings. During the passage of the gas over the magnesium at a bright red heat, the nitrogen is absorbed in a great degree, and the gas which finally passes through is immensely richer in argon than that which first enters the hot tube.[34] At the present time you see a tolerably rapid bubbling on the left, indicative of the flow of atmospheric nitrogen into the combustion furnace; whereas, on the right, the outflow is very much slower. Care must be taken to prevent the heat rising to such a point as to soften the glass. The concentrated argon is collected in a second gas-holder, and afterwards submitted to further treatment.[35] The apparatus employed by Professor Ramsay in the subsequent treatment is exhibited in the diagram, and is very effective for its purpose; but I am afraid that the details of it would not readily be followed from any explanation that I could give in the time at my disposal. The principle consists in the circulation of the mixture of nitrogen and argon over hot magnesium, the gas being made to pass round and round until the nitrogen is effectively removed from it. At the end that operation, as in the case of the oxygen method, proceeds somewhat slowly.[36] When the greater part of the nitrogen is gone, the remainder seems to be unwilling to follow, and it requires somewhat protracted treatment in order to be sure that the nitrogen has wholly disappeared. When I say "wholly disappeared," that perhaps, would be too much to say in any case. What we can say is that the spectrum test is adequate to show the presence, or at any rate to show the addition, of about one-and-a-half per cent. of nitrogen to argon as pure as we can get it; so that it is fair to argue that any nitrogen at that stage remaining in the argon is only a small fraction of one-and-a-half per cent.[37]
I should have liked at this point to be able to give advice as to which of the two methods--the oxygen method or the magnesium method--is the easier and the more to be recommended; but I confess that I am quite at a loss to do so. One difficulty in the comparison arises from the fact that they have been in different hands. As far as I can estimate, the quantities of nitrogen eaten up in a given time are not very different. In that respect, perhaps, the magnesium method has some advantage; but, on the other hand, it may be said that the magnesium process requires a much closer supervision, so that, perhaps, fourteen hours of the oxygen process may not unfairly compare with eight hours or so of the magnesium method. In practice a great deal would depend upon whether in any particular laboratory alternate currents are available from a public supply. If the alternate currents are at hand, I think it may probably be the case that the oxygen method is the easier; but, otherwise, the magnesium method would, probably, be preferred, especially by chemists who are familiar with operations conducted in red-hot tubes.[38]
I have here another experiment illustrative of the reaction between magnesium and nitrogen. Two rods of that metal are suitably mounted in an atmosphere of nitrogen, so arranged that we can bring them into contact and cause an electric arc to form between them. Under the action of the heat of the electric arc the nitrogen will combine with the magnesium; and if we had time to carry out the experiment we could demonstrate a rapid absorption of nitrogen by this method. When the experiment was first tried, I had hoped that it might be possible, by the aid of electricity, to start the action so effectively that the magnesium would continue to burn independently under its own developed heat in the atmosphere of nitrogen. Possibly, on a larger scale, something of this sort might succeed, but I bring it forward here only as an illustration. We turn on the electric current, and bring the magnesiums together. You see a brilliant green light, indicating the vaporisation of the magnesium. Under the influence of heat the magnesium burns, and there is collected in the glass vessel a certain amount of brownish-looking powder which consists mainly of the nitride of magnesium.[39] Of course, if there is any oxygen present it has the preference, and the ordinary white oxide of magnesium is formed.[40]
The gas thus isolated is proved to be inert by the very fact of its isolation. It refuses to combine under any circumstances in which nitrogen, itself always considered very inert, does combine--both in the case of the oxygen treatment and in the case of the magnesium treatment; and these facts are, perhaps, almost enough to justify the name which we have suggested for it.[41] But in addition to this, it has been proved to be inert under a considerable variety of other conditions such as might have been expected to tempt it into combination. I will not recapitulate all the experiments which have been tried, almost entirely by Professor Ramsay, to induce the gas to combine.[42] Hitherto, in our hands, it has not done so; and I may mention that recently, since the publication of the abstract of our paper read before the Royal Society, argon has been submitted to the action of titanium at red heat, titanium being a metal having a great affinity for nitrogen, and that argon has resisted the temptation to which nitrogen succumbs. We never have asserted, and we do not now assert, that argon can under no circumstances be got to combine. That would, indeed, be a rash assertion for any one to venture upon; and only within the last few weeks there has been a most interesting announcement by M. Berthelot, of Paris, that, under the action of the silent electric discharge, argon can be absorbed when treated in contact with the vapour of benzine.[43] Such a statement, coming from so great an authority, commands our attention; and if we accept the conclusion, as I suppose we must do, it will follow that argon has, under those circumstances, combined.
Argon is rather freely soluble in water. That is a thing that troubled us at first in trying to isolate the gas; because, when one was dealing with very small quantities, it seemed to be always disappearing.[44] In trying to accumulate it we made no progress. After a sufficient quantity had been prepared, special experiments were made on the solubility of argon in water. It has been found that argon, prepared both by the magnesium method and by the oxygen method, has about the same solubility in water as oxygen--some two-and-a half times the solubility of nitrogen. This suggests, what has been verified by experiment, that the dissolved gases of water should contain a larger proportion or argon than does atmospheric nitrogen. I have here an apparatus of a somewhat rough description, which I have employed in experiments of this kind. The boiler employed consists of an old oil-can. The water is supplied to it and drawn from it by coaxial tubes of metal. The incoming cold water flows through the outer annulus between the two tubes. The outgoing hot water passes through the inner tube, which ends in the interior of the vessel at a higher level. By means of this arrangement the heat of the water which has done its work is passed on to the incoming water not yet in operation, and in that way a limited amount of heat is made to bring up to the boil a very much larger quantity of water than would otherwise be possible,[45] the greater part of the dissolved gases being liberated at the same time. These are collected in the ordinary way. What you see in this flask is dissolved air collected out of water in the course of the last three or four hours. Such gas, when treated as if it were atmospheric nitrogen, that is to say after removal of the oxygen and minor impurities, is found to be decidedly heavier than atmospheric nitrogen to such an extent as to indicate that the proportion of argon contained is about double.[46] It is obvious, therefore, that the dissolved gases of water form a convenient source of argon, by which some of the labor of separation from air is obviated. During the last few weeks I have been supplied from Manchester by Mr Macdougall, who has interested himself in this matter, with a quantity of dissolved gases obtained from the condensing water of his steam engine.
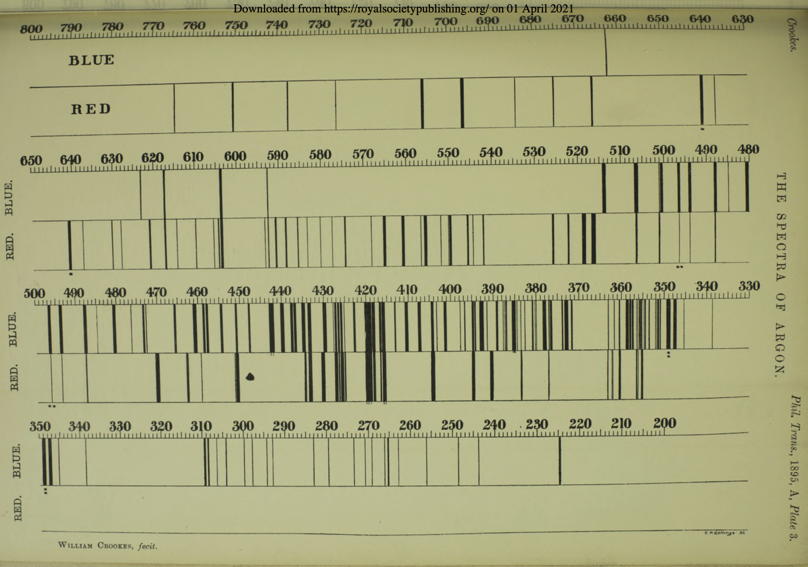
As to the spectrum,[47] we have been indebted from the first to Mr Crookes, and he has been good enough to-night to bring some tubes which he will operate, and which will show you at all events the light of the electric discharge in argon. I cannot show you the spectrum of argon, for unfortunately the amount of light from a vacuum tube is not sufficient for the projection of its spectrum. Under some circumstances the light is red, and under other circumstances it is blue. Of course when these lights are examined with the spectroscope--and they have been examined by Mr Crookes with great care--the differences in the colour of the light translate themselves into different groups of spectrum lines. We have before us Mr Crookes' map, showing the two spectra upon a very large scale. The upper is the spectrum of the blue light; the lower is the spectrum of the red light; and it will be seen that they differ very greatly. Some lines are common to both; but a great many lines are seen only in the red, and others are seen only in the blue. It is astonishing to notice what trifling changes in the conditions of the discharge bring about such extensive alterations in the spectrum.
One question of great importance upon which the spectrum throws light is, Is the argon derived from the oxygen method really the same as the argon derived by the magnesium method?[48] By Mr Crookes' kindness I have had an opportunity of examining the spectra of the two gases side by side, and such examination as I could make revealed no difference whatever in the two spectra, from which, I suppose, we may conclude either that the gases are absolutely the same, or, if they are not the same, that at any rate the ingredients by which they differ cannot be present in more than a small proportion in either of them.
My own observations upon the spectrum have been made principally at atmospheric pressure. In the ordinary process of sparking, the pressure is atmospheric; and, if we wish to look at the spectrum, we have nothing more to do than to include a jar in the circuit, and to put a direct-vision prism to the eye. At my request, Professor Schuster examined some tubes containing argon at atmospheric pressure prepared by the oxygen method, and I have here the diagram of a characteristic group. He also placed upon the sketch some of the lines of zinc, which were very convenient as directing one exactly where to look. See figure.
![[drawing of line spectra of argon, zinc and hydrogen]](RAYLEIGH2.jpg)
Within the last few days, Mr Crookes has charged a radiometer with argon. When held in the light from the electric lamp, the vanes revolve rapidly.[49] Argon is anomalous in many respects, but not, you see, in this.
Next, as to the density of argon. Professor Ramsay has made numerous and careful observations upon the density of the gas prepared by the magnesium method, and he finds a density of about 19.9 as compared with hydrogen.[50] Equally satisfactory observations upon the gas derived by the oxygen method have not yet been made, but there is no reason to suppose that the density is different, such numbers as 19.7 having been obtained.
One of the most interesting matters in connection with argon, however, is what is known as the ratio of the specific heats. I must not stay to elaborate the questions involved, but it will be known to many who hear me that the velocity of sound in a gas depends upon the ratio of two specific heats--the specific heat of a gas measured at constant pressure, and the specific heat measured at constant volume.[51] If we know the density of a gas, and also the velocity of sound in it, we are in a position to infer this ratio of specific heats; and by means of this method, Professor Ramsay has determined the ratio in the case of argon, arriving at the very remarkable result that the ratio of specific heats is represented by the number 1.65, approaching very closely to the theoretical limit, 1.67. The number 1.67 would indicate that the gas has no energy except energy of translation of its molecules. If there is any other energy than that, it would show itself by this number dropping below 1.67.[52] Ordinary gases, oxygen, nitrogen, hydrogen, &c., do drop below, giving the number 1.4. Other gases drop lower still. If the ratio of specific heats is 1.65, practically 1.67, we may infer that the whole energy of motion is translational; and from that it would seem to follow by arguments which, however, I must not stop to elaborate, that the gas must be of the kind called by chemists monatomic.
I had intended to say something of the operation of determining the ratio of specific heats, but time will not allow. The result is, no doubt, very awkward. Indeed, I have seen some indications that the anomalous properties of argon are brought as a kind of accusation against us. But we had the very best intentions in the matter. The facts were too much for us; and all that we can do now is to apologise for ourselves and for the gas.[53]
Several questions may be asked, upon which I should like to say a word or two, if you will allow me to detain you a little longer.[54] The first question (I do not know whether I need ask it) is, Have we got hold of a new gas at all? I had thought that that might be passed over, but only this morning I read in a technical journal the suggestion that argon was our old friend nitrous oxide. Nitrous oxide has roughly the density of argon; but that, so far as I can see, is the only point of resemblance between them.[55]
Well, supposing that there is a new gas, which I will not stop to discuss, because I think that the spectrum alone would be enough to prove it, the next question that may be asked is, Is it in the atmosphere?[56] This matter naturally engaged our earnest attention at an early stage of the enquiry. I will only indicate in a few words the arguments which seem to us to show that the answer must be in the affirmative.
In the first place, if argon be not in the atmosphere, the original discrepancy of densities which formed the starting-point of the investigation remains unexplained, and the discovery of the new gas has been made upon a false clue. Passing over that, we have the evidence from the blank experiments, in which nitrogen originally derived from chemical sources is treated either with oxygen or with magnesium, exactly as atmospheric nitrogen is treated. If we use atmospheric nitrogen, we get a certain proportion of argon, about 1 per cent. If we treat chemical nitrogen in the same way we get, I will not say absolutely nothing, but a mere fraction of what we should get had atmospheric nitrogen been the subject.[57] You may ask, Why do we get any fraction at all from chemical nitrogen? It is not difficult to explain the small residue, because in the manipulation of the gases large quantities of water are used; and, as I have already explained, water dissolves argon somewhat freely. In the processes of manipulation some of the argon will come out of solution and it remains after all the nitrogen has been consumed.
Another wholly distinct argument is founded upon the method of diffusion introduced by Graham[58]. Graham showed that if you pass gas along porous tubes you alter the composition, if the gas is a mixture. The lighter constituents go more readily through the pores than do the heavier ones. The experiment takes this form. A number of tobacco pipes--eight in the actual arrangement--are joined together in series with indiarubber junctions, and they are put in a space in which a vacuum can be made, so that the space outside the porous pipes is vacuous or approximately so. Through the pipes ordinary air is led. One end may be regarded as open to the atmosphere. The other end is connected with an aspirator so arranged that the gas collected is only some 2 per cent. of that which leaks through the porosities. The case is like that of an Australian river drying up almost to nothing in the course of its flow. Well, if we treat air in that way, collecting only the small residue which is less willing than the remainder to penetrate the porous walls, and then prepare "nitrogen" from it by removal of oxygen and moisture, we obtain a gas heavier than atmospheric nitrogen, a result which proves that the ordinary nitrogen of the atmosphere is not a simple body, but is capable of being divided into parts by so simple an agent as the tobacco pipe.[59]
If it be admitted that the gas is in the atmosphere, the further question arises as to its nature.[60]
At this point I would wish to say a word of explanation. Neither in our original announcement at Oxford, nor at any time since, until the 31st of January, did we utter a word suggesting that argon was an element; and it was only after the experiments upon the specific heats that we thought that we had sufficient to go upon in order to make any such suggestion in public. I will not insist that the observation is absolutely conclusive. It is certainly strong evidence.[61] But the subject is difficult, and one that has given rise to some difference of opinion among physicists. At any rate this property distinguishes argon very sharply from all the ordinary gases.[62]
One question which occurred to us at the earliest stage of the enquiry, as soon as we knew that the density was not very different from 21, was the question of whether, possibly, argon could be a more condensed form of nitrogen, denoted chemically by the symbol N3.[63] There seem to be several difficulties in the way of this supposition. Would such a constitution be consistent with the ratio of specific heats (1.65)? That seems extremely doubtful. Another question is, Can the density really be as high as 21, the number required on the supposition of N3? As to this matter, Professor Ramsay has repeated his measurements of density, and he finds that he cannot get even so high as 20. To suppose that the density of argon is really 21, and that it appears to be 20 in consequence of nitrogen still mixed with it, would be to suppose a contamination with nitrogen out of all proportion to what is probable. It would mean some 14 per cent. of nitrogen, whereas it seems that from one-and-a-half to two per cent. is easily enough detected by the spectroscope. Another question that may be asked is, Would N3 require so much cooling to condense it as argon requires?
There is one other matter on which I would like to say a word--the question as to what N3 would be like if we had it. There seems to be a great discrepancy of opinions. Some high authorities, among whom must be included, I see, the celebrated Mendeleef, consider that N3 would be an exceptionally stable body; but most of the chemists with whom I have consulted are of the opinion that N3 would be explosive, or, at any rate, absolutely unstable.[64] That is a question which may be left for the future to decide. We must not attempt to put these matters too positively. The balance of evidence still seems to be against the supposition that argon is N3, but for my part I do not wish to dogmatise.
A few weeks ago we had an eloquent lecture from Professor Rücker on the life and work of the illustrious Helmholtz.[65] It will be known to many that during the last few months of his life Helmholtz lay prostrate in a semi-paralysed condition, forgetful of many things, but still retaining a keen interest in science. Some little while after his death we had a letter from his widow, in which she described how interested he had been in our preliminary announcement at Oxford upon this subject, and how he desired the account of it to be read to him over again. He added the remark, "I always thought that there must be something more in the atmosphere."
[2]The discovery described in this lecture evolved from a long and meticulous program of research Rayleigh began over a decade earlier. He had turned his attention to the measurement of the density and atomic weight of the principal gases of the atmosphere. His object was to obtain measurements as accurate as possible to test Prout's multiples hypothesis. (See chapter 10.) Accurate measurements seemed to show that the atomic weights of many if not most elements were approximately, but not exactly, whole-number multiples of that of hydrogen. Rayleigh began his measurements by examining the ratio of densities and atomic weights of oxygen and hydrogen, reporting the results of these measurements in the late 1880s and early 1890s [Rayleigh 1892a]. He then turned to nitrogen.
[3]For over a century, atmospheric air had been known to be a mixture of gases including nitrogen (the most plentiful constituent) and oxygen (the constituent which supports respiration and combustion). Rayleigh attempted to prepare nitrogen by removing the other gases from atmospheric air. The reaction of oxygen (O2) with ammonia (NH3) may be represented as:
3 O2 + 4 NH3 --> 6 H2O + 2 N2 .Thus, reaction with excess ammonia gets rid of the oxygen. The nitrogen (N2) produced in this reaction remains in the gas sample, along with the nitrogen originally present in the air. Any left-over ammonia is "absorbed" (neutralized) by acid, which removes it from the gas sample. Water vapor (H2O) is removed from the gas by letting it pass over solids which absorb it ("ordinary desiccating agents") such as calcium chloride or silica gel.
[4]That is, Rayleigh had prepared a number of samples by this method, and found their densities to be reproducible and consistent.
[5]Why should Rayleigh use more than one method? And if he was going to use an older method anyway, why had he used Harcourt's method in the first place? In effect, by using Harcourt's method and comparing it to an older method, he was trying out a new tool or method for removing oxygen from a mixture of gases. It is often useful for scientists to have more than one method for accomplishing a particular task, in case one method does not work under certain circumstances. (For example, in investigating a gas which reacts with copper, it would not be appropriate to use the older method of removing oxygen, which involved copper; in that case, it would be useful to have another method to draw upon.) The ammonia method was based on a known reaction, and Rayleigh had every reason to believe that it would work; however, it was useful to see just how well it did work by comparing its results against another, established method.
[6]The traditional method used the reaction:
O2 + 2 Cu --> 2 CuO .The resulting copper oxide is a solid, so it does not stay with the gas sample.
[7]Note how small the discrepancy was. A less careful scientist might not notice such a discrepancy. Indeed, there are many cases in which a difference of 0.1% in a pair of results is "close enough." When is such a small difference worth pursuing? When the methods used in the experiment are capable of measuring to greater precision than the difference. How can one tell whether the method is so precise? One would have to do what Rayleigh did: carry out several trials using each method. If the results from each method agreed with each other to within, say, 0.02%, then results which differed by 0.1% would correctly be regarded as slightly different results. Statistical analysis of multiple measurements by different methods provides more formal criteria for judging the likelihood that an apparent difference between methods is an actual difference.
[8]Rayleigh is now trying to find an explanation of the difference. At this point he probably expected to find that one method or the other was somehow not quite reliable for isolating nitrogen, and he wanted to find out which method was at fault and in what way. In fact, finding an unexpected or overlooked flaw in one of the methods--perhaps correctable, perhaps not--is the usual result of tracking down such discrepancies. In other words, a careful and successful explanation of such a difference would not usually result in a major discovery, but only in determining why a proposed new method doesn't work or at most in discovering a way to make that new experimental method useful after all. A curious and scrupulous scientist knows that even diligent work in tracking down anomalies and discrepancies is usually rewarded with only modest results, often not even worth publishing; he or she also knows that important discoveries cannot be made unless such anomalies are pursued.
Troubleshooting (for that is what the pursuit of anomalies in a new experimental method amounts to) generally calls for an approach much like the traditional version of the scientific method on a small scale. One can imagine Rayleigh formulating possible explanations for the discrepancy (hypotheses) and devising ways to test those explanations (experiments). For example, maybe Harcourt's method did not remove all the oxygen. How might Rayleigh have tested this possibility? Well, if some oxygen remained in the sample, then the sample should have a higher density than that measured by the old method, for oxygen is denser than nitrogen. Or he could have run the reaction with more ammonia or for a longer time: if Harcourt's method had removed all the oxygen, than more ammonia or more time could not remove more oxygen and would not change the results.
[9]See Rayleigh 1892b. Publishing an article which describes puzzling results is not a common occurrence, especially today. Reputable scientific journals are very selective in what they publish, and they prefer finished work. A puzzled scientist turning to others for help, particularly others with different areas of expertise, is not unusual--although it is unusual to see it publicly and in print. Rayleigh was a physicist. The methods he had used involved chemical reactions, and he sought the advice of chemists as to what might have happened and how to probe what did happen.
Note that this section of Rayleigh's talk displays language that would not have appeared in a typical scientific article of his time or of ours. Feelings such as disgust and impatience are left out of journal articles scientists write for other scientists in their field. Such articles are confined to what the researchers did, what they found, and how they made sense of what they found. Why they did what they did how how they felt when they did it are historically interesting, but they are typically left out of scientists' professional communications to each others. Recall that this article is a transcript of a public lecture, not of a formal communication intended only for other scientists.
[10]Atmospheric air contains roughly 80% nitrogen and 20% oxygen by volume, and this was known to Rayleigh. So out of every 100 air molecules in his original sample, 80 were nitrogen and 20 oxygen. Since the ammonia method produces two N2 molecules for every three O2 molecules removed from the air, nearly 14 nitrogen molecules would replace the 20 oxygens. Thus, out of the roughly 94 nitrogen molecules now present, 14 of them (or about one in seven) were produced by the ammonia-oxygen reaction, the remainder having been present all along in the atmosphere.
[11]Rayleigh wondered whether the nitrogen produced from the chemical reaction might be different from the nitrogen of the atmosphere. Nitrogen gas consists of two atoms of nitrogen bonded together, denoted N2. In particular, if some of the nitrogen from the ammonia method were in the form of free atoms, non-bonded N, that would make the sample less dense than a sample which contained only N2 because some N atoms would replace N2 molecules. If the isolated atoms stayed isolated (which in fact they don't do; they react with each other), they would take up the same amount of space as N2 molecules but weigh only half as much.
[12]Of course when the purpose of the investigation was to measure the weight of nitrogen, Rayleigh would have wished that there was no discrepancy between the two methods. But once he determined that there was a real and persistent (albeit small) discrepancy, he made the discrepancy itself the subject of his continued inquiry. So it makes sense to magnify or amplify the object of his study in order to "see" it better.
Exaggerating or amplifying such a discrepancy is sometimes easier said than done. How can one amplify an effect (the discrepancy) whose cause is unknown? A logical place to begin is in looking for differences between the two methods, and to try to exaggerate those differences, preferably one at a time. This is exactly what Rayleigh did in focusing on the nitrogen produced from ammonia in the ammonia method.
[13]Comparing the N2 obtained from a variety of methods and conditions would allow Rayleigh to determine if any of the methods was unreliable. The fact that all the "chemical nitrogen" measurements agree is good evidence that all the methods produce the same thing, namely pure N2.
Details of these experiments were published in Rayleigh 1893 and Rayleigh 1894. In all the experiments reported in the tables, Rayleigh prepared a sample of gas, filled a glass globe with it at a specified temperature and pressure, and weighed the gas-filled globe. The entries labeled atmospheric nitrogen were all prepared by removing oxygen from a sample of atmospheric air. Two methods removed oxygen by reacting it with hot metal, either copper (described above) or iron (Fe)
3 O2 + 4 Fe --> 2 Fe2O3 .The method labeled ferrous hydrate was done at room temperature, and involved bubbling air through a solution of ferrous sulfate (FeSO4) and calcium hydroxide (Ca(OH)2):
O2 + 4 FeSO4 + 4 Ca(OH)2 --> 2 Fe2O3 + 4 CaSO4 + 4 H2O .The entries labeled chemical nitrogen were prepared not from atmospheric air, but from different nitrogen-containing substances, namely the gaseous oxides of nitrogen nitric oxide (NO) or nitrous oxide (N2O), ammonium nitrite (NH4NO2, a solid), and urea ((NH2)2CO, a water-soluble solid present in urine). The first two release their nitrogen when reacted with hot iron:
6 NO + 4 Fe --> 3 N2 + 2 Fe2O3 ,Chemists would say that the nitrogen in these compounds was reduced to N2. The reaction of urea ((NH2)2CO) with sodium hypochlorite (NaOCl) was suggested to Rayleigh as a method that would not require hot iron,
3 N2O + 2 Fe --> 3 N2 + Fe2O3 .
(NH2)2CO + 3 NaOCl --> N2 + CO2 + 2 H2O + 3 NaCl .Here the nitrogen in urea is oxidized to N2. (Likewise for the nitrogen in ammonia in Rayleigh's earlier experiment.) Ammonium nitrite decomposes upon heating, releasing nitrogen and water vapor:
NH4NO2 --> N2 + 2 H2O .In this reaction, one nitrogen-containing fragment is oxidized and the other reduced, to yield N2.
[14]The data from the tables are presented in the graph below. The graph illustrates even more obviously than the numbers the real--albeit small--difference between "atmospheric nitrogen" and "chemical nitrogen."
![[bar chart of chemical and atmospheric nitrogen measurements]](RAYLEIGH1.gif)
[15]Experimenters in every branch of the natural sciences--not just chemistry--must be careful to guard against impurities. Small amounts of particular substances can poison organisms in biological research or "poison" reactions in chemistry. Even inert impurities can affect results because part of the sample believed to be one substance is actually something else (namely the impurity). Here Rayleigh is particularly concerned about hydrogen, the lightest of the gases, with a density 1/14 that of nitrogen.
[16]Rayleigh thought the possibility that some of the nitrogen was in the form of free atoms (non-bonded N) unlikely, because his chemist colleagues told him that free N atoms are very reactive. They tend to combine very rapidly with other reactive species including other N atoms; that is to say, if any free N atoms were produced in the chemical preparation of nitrogen, in all likelihood they would quickly have linked up with each other to produce N2.
[17]Even though he had reason to be skeptical of the hypothesis that some of his "chemical nitrogen" contained appreciable numbers of free N atoms, Rayleigh tested the possibility by preparing "chemical nitrogen," weighing a sample of it in his standard glass globe under controlled temperature and pressure, and then weighing a sample of it again after eight months under the same conditions. The idea here is that if any N atoms were present during the first weighing of the sample, they surely would have paired up during the next eight months; the second weighing would then contain only N2. So if the second sample weighed more than the first, that would support the notion that during the first weighing there were free N atoms present. As it turned out, though, there was no detectable difference in weight after eight months, suggesting that there were no free N atoms even at first.
As Rayleigh notes, however, he did not simply shut a sample up in the globe for eight months. The only way recombination of N atoms would be detectable, if it had occurred, would be to fill the globe from a large sample of gas, weigh it, return it to the sample, let the sample sit for eight months, and then refill the globe from the sample and reweigh it. If the N atoms recombined, then there would be slightly more room in the globe to let in more N2 molecules than had fit during the first weighing.
[18]Here Rayleigh attempted to break apart N2 molecules by means of an electric discharge (spark) long enough to detect a lowering of density due to free N atoms. He was unable to detect any differences in weight before and after the sparking in either the atmospheric or chemical nitrogen samples. Presumably this is because free N atoms would persist under the conditions of his experiment for only a fraction of a second, not long enough to weigh them once they were formed. However, it is also possible that Rayleigh did not produce any free N atoms in the first place, for it takes a great deal of energy to pry apart the bonds which hold N2 together.
[19]Henry Cavendish (1731-1810; see portrait at National Portrait Gallery, London) made a number of important contributions to chemistry and physics in the late 18th century. He measured the mass and density of the earth, and was the first to measure Newton's constant of universal gravitation. In chemistry, he concerned himself mainly with gases, such as Cavendish 1785, the paper referred to here. He was the first to recognize hydrogen ("inflammable air" as he called it) as a distinct substance, and was among the first to discover that water was not an element (chapter 6). He also found that the ratio of nitrogen to oxygen in air is the same near the surface of the earth as it is higher in the atmosphere (reachable then by balloon).
[20]Until the 17th century, air was thought to be a single substance, rather than a mixture. Once air was recognized to be a mixture, the part which was necessary for animals to breathe was distinguished from the rest [Mayow 1674], which was mainly nitrogen. Thus, one of the characteristics of nitrogen gas was its inertness and lifelessness (both literally, since it did not sustain life, and figuratively, since it reacted chemically only under rather extreme conditions such as sparks and flames). In fact, this lifelessness is reflected in the French name for nitrogen, azote. Nitre is a nitrogen-containing mineral used in making gunpowder; its chemical name is potassium nitrate (KNO3). In Cavendish's time, it was known that nitre contained nitrogen or something similarly lifeless.
[21]A schematic diagram of Cavendish's apparatus appears below. Since mercury is a metal, it conducts electricity from the electrical source to the gases; since it is a liquid in which the gases do not dissolve, gases can conveniently be introduced into the top of the apparatus by bubbling them through one of the tube's legs; and since it is a dense liquid, the basic substance Rayleigh calls potash will float upon it, maintaining contact with the gases. (Potash can refer to either potassium carbonate or potassium hydroxide. Both substances are basic, but the latter is much more caustic. Since Cavendish refers to the substance as soap-lees, he probably used potassium hydroxide, also known as lye, which could be used in making soap.) With the help of the sparks, the nitrogen in the tube reacts with the oxygen, producing corrosive gases such as NO and NO2, the chemical precursors of nitric acid. Potash reacts with these nitrogen oxides and thereby removes them from the gas ("absorbs" them). As the gases react, the volume of gas diminishes, and mercury rises in the tube to replace the gases. Thus the figure represents the apparatus after the experiment has been operating a while.
![[figure from Cavendish paper]](../cav4.gif)
The figure above is from Cavendish 1785. The figure below is a modification of Cavendish's; it illustrates the mercury risen partway up the tube after the reaction has run for a while.
![[figure adapted and altered from Cavendish paper]](cav4after.gif)
[22]Rayleigh does not mention that Cavendish proceeded to examine the potash afterwards. After drying it, he found nitre in it. Thus the nitrogen of the atmosphere was converted into nitre.
[23]Cavendish kept the electricity running until the amount of gas stopped decreasing. He had put an excess of oxygen in the mixture along with atmospheric air, so in effect, he kept the electricity running until all the nitrogen was reacted. He removed ("absorbed") the remaining oxygen. The small residue left over must have been argon at least in part: Cavendish had very likely isolated or at least concentrated argon over a hundred years earlier than Rayleigh.
Cavendish had neither the inclination nor the tools to pursue the matter. Indeed, it made no sense at the time to characterize minor components of the atmosphere before major ones were well understood. Furthermore, it would have taken a considerable effort for Cavendish to ascertain that the residue was even reproducible (that is, real). But that was not his purpose; seeing that the residue was minor told him that practically all the "inert" part of the atmosphere was accounted for in the form of nitrogen.
[24]Rayleigh began to collaborate with a chemist, William Ramsay (1852-1916). Ramsay was awarded the Nobel Prize in chemistry in 1904 for his work on the discovery of a whole family of inert gases, of which argon was the first discovered. We will hear from Ramsay in the next chapter. Their formal report of the discovery of argon to the scientific community is found in Rayleigh & Ramsay 1895.
[25]This casual remark illustrates one of the important connections between science (which is concerned with gaining knowledge about the world) and technology (which is concerned with making tools). People of the early 21st century are quite aware fact that they inhabit a highly technological world, and that scientific knowledge provides the basis for many technological innovations. It is equally true, but less widely appreciated, that technological improvement can assist the generation of scientific knowledge. Here, for example, we can take note of the advance in electrical technology that had taken place between Cavendish's time and Rayleigh's. Cavendish had to use "friction machines" to produce electrical sparks. These devices generated static electrical charges by rubbing together materials such as silk and amber; there were no controlled electric currents to speak of, only discharge of these static charges. In the intervening time, voltaic cells or batteries (direct current devices), as well as dynamos or generators (alternating current devices) were developed, along with devices such as transformers which could manipulate these currents. Rayleigh's comparatively advanced electrical technology allowed him to do as a lecture demonstration what Cavendish could accomplish only with great difficulty; and we will see that Rayleigh could scale up his apparatus to produce large quantities of argon. (Note, by the way, that electricity was still far from a universal household convenience even to Rayleigh: he later points out that "this Institution," in contrast to many places, has a "public supply" of electricity generated at Deptford, a few miles to the southeast of the Royal Institution.)
[26]Heinrich Rühmkorff's improvements to the induction coil allowed late 19th-century scientists to use high voltages (up to about 100,000 V) and therefore high energies in their experiments. The Rühmkorff coil was an important piece of equipment in investigations which led to the discovery of electromagnetic waves, X-rays, and the electron. William Robert Grove was a lawyer and judge who did important work in electrochemistry, including developing the electrochemical cell named after him. The Grove cell provides electrical current from the chemical reaction of zinc with the nitrate ion
Zn + H2SO4 + 2 HNO3 --> ZnSO4 + 2 H2O + 2 NO2 .A battery of cells consisted of several electrochemical cells connected together. Note that this usage is different from our common usage today, whereby the word battery usually refers to a single electrochemical cell.
[27]In his apparatus, Rayleigh substitutes an alkaline (i.e., basic) liquid for the floating piece of potash: it also floats atop the mercury and removes the oxides of nitrogen from the gas mixture.
[28]Rayleigh notes that the apparatus works well enough for the purpose of demonstrating that some gaseous residue is left over after all the nitrogen reacts. But if one is interested in isolating that residue for further study, one must "scale up" the process. Rayleigh spends a fair amount of time describing his apparatus. This would be important for anyone interested in repeating his work, and in principle such replication is an important part of the development of scientific knowledge. That is, the results of scientific investigation must be open to verification (or falsification, as the case may be). The technical details of his improved apparatus, however, are not vitally important to our assessment of Rayleigh's methodology.
[29]The British scientist Sir William Crookes (1832-1919; see portrait at National Portrait Gallery, London) was a productive researcher and highly original and speculative thinker in many areas of physics and chemistry. For example, he invented the radiometer (mentioned later), and worked with electric discharges and spectra. He was a pioneer in the field of spectroscopy, which helped him discover the element thallium (element 81). His work in chemistry also involved a painstaking study of the rare earth elements. Crookes was important not only as a scientist but also as a communicator of science. He was the founder and long-time editor of the Chemical News. He was not afraid to be wrong; his bent for speculation led both to productive developments and to blind alleys. The mention of Crookes or of his inventions in this collection of selections on the periodic law, the discovery of argon and the search for related elements, the discovery of the electron, and the discovery of radioactivity (chapters 13 through 17) attests to the breadth of his interests and influence.
[30]Here is a drawing of Rayleigh's apparatus from Rayleigh and Ramsay 1895:
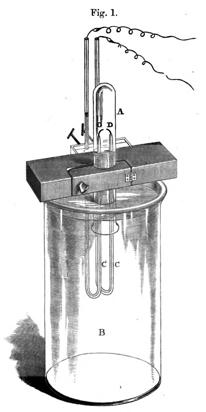
[31]The description of apparatus is illustrative of the way in which technological or engineering expertise was needed in order to put together the apparatus. Here and below Rayleigh mentions heat buildup (and therefore the need for parts that can withstand and/or disperse such heat); below he discusses keeping the apparatus free from leaks, and the necessity of using materials such as glass which stand up to strong acids and bases.
[32]To "approximate" means to "bring near." Rayleigh says the points (sharp electrical terminals) must be brought near each other to make the spark jump across a small gap. Once the spark is struck, the terminals can be moved apart somewhat.
[33]For example, by reaction with hot copper, as mentioned above.
[34]Magnesium (Mg) is quite a reactive metal, and when red-hot it will react even with relatively inert nitrogen gas to form the solid magnesium nitride (Mg3N2):
3 Mg + N2 --> Mg3N2 .Thus the magnesium seems to "absorb" the nitrogen, removing it from the gas and incorporating it into the solid.
[35]That is, after making a first pass over the hot magnesium, the remaining gas is collected for further purification (for it still contains some nitrogen). The further treatment is essentially more of the same: repeated circulation over hot magnesium. Here is a drawing of Ramsay's apparatus from Rayleigh and Ramsay 1895:
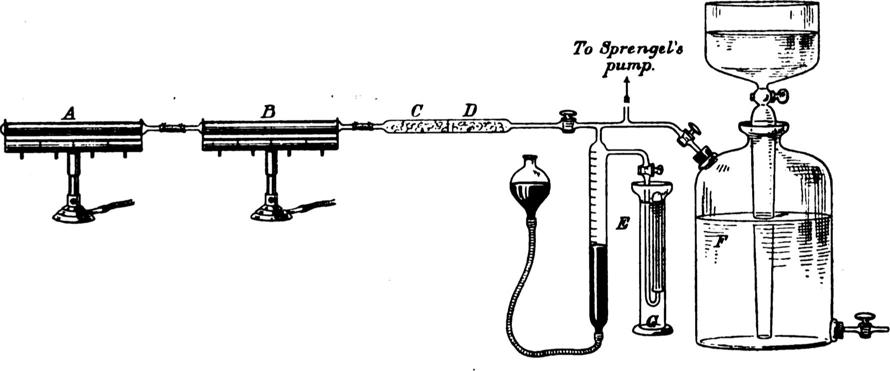 .
.
[36]Purification and separation processes are generally difficult at the end: it is more difficult to remove the last traces of contamination.
[37]That is, when the treatment is complete, the test shows nothing but argon. That in itself, is enough to say that there is little if any contamination along with the argon. But Rayleigh provides us with another piece of information: if he adds 11/2% nitrogen to the sample of argon, even that small added amount does show up in the same test. Thus we have some idea of the maximum amount of contamination possible in the original sample: considerably less than 11/2%.
[38]The industrial preparation and purification of argon today uses neither of these processes. Instead, atmospheric air is refrigerated to very cold temperatures, and its various components are condensed at their boiling temperatures, a process called fractional distillation. That is, the various gases become liquid at characteristic temperatures; as each component is liquefied, it is separated from those which remain gaseous. Indeed, this process was used to prepare argon even within a few years of its discovery. Argon purified in this way was widely used to fill incandescent light bulbs, for filaments do not burn out as quickly when they operate surrounded by an unreactive gas. Argon is also commonly used as an inert insulator between the panes of double-glazed windows.
[39]Rayleigh hoped that the reaction between magnesium and nitrogen would continue on its own once it was initiated, for example by an electric spark. The thought was a reasonable one, for the reaction does give off quite a bit of heat, and it was reasonable to hope that that heat could make the relatively unreactive nitrogen keep reacting. After all, there are a number of reactions which need a spark or flame to get them started, but which then continue on their own. In fact, many such reactions involve burning, that is the vigorous combination of a reactant with oxygen; in analogy to such reactions, Rayleigh speaks of hoping magnesium would "burn" in nitrogen. Of course if he were able to produce a self-sustaining reaction, he would not need to use so much electricity or heat from external sources to keep the reaction going.
[40]Oxygen is much more reactive than nitrogen, so if any oxygen is present, the magnesium reacts with it first:
2 Mg + O2 --> 2 MgO .The product, magnesium oxide (MgO) is a white powder.
[41]The name argon was coined from the Greek α-εργον (a-ergov) meaning slow or lazy--literally, unenergetic. At the time of its discovery, its inertness was its most striking characteristic: argon was isolated because it remained inert under extreme conditions under which even nitrogen reacted. Today, an entire column of the periodic table consists of relatively unreactive gases, but argon was the first of them to be isolated and was the first clue that its relatives existed.
[42]Put yourselves in the place of Rayleigh and Ramsay. Suppose you have just discovered a gas previously unknown, and you have a means of producing as much of it as you wish. Naturally, you would wish to find out more about it, to characterize it. What might you try to learn about it? A logical place to start would be with the one property you have already glimpsed in the process of preparing argon, its unreactiveness: under what circumstances, if any, can argon be made to react (combine) with other chemicals? Another important characteristic would be its density, which is after all what Rayleigh was set up to measure for nitrogen and which can shed light on the possible molecular formula of the new substance, which was not yet known to be an element. Rayleigh will touch on this question below. In the meantime, however, he also mentions the solubility of argon and its spectrum--two more characteristics of the new gas.
[43]Ramsay mixed argon with a wide variety of elements, acids, bases, oxidants, and reducing agents. Argon failed to react with hydrogen, chlorine, phosphorous vapor, sulfur vapor, tellurium vapor, sodium vapor, molten sodium hydroxide, molten potassium nitrate, potassium permanganate in hydrochloric acid, sodium peroxide, bromine water, and a cocktail of nitric and hydrochloric acids among others [Rayleigh & Ramsay 1895]. For all practical purposes, argon has still not been made to combine chemically with itself or with anything else. (Indeed, its first stable neutral compound, argon fluorohydride (HArF), was not reported until 2000, and it exists only in a low-temperature solid matrix [Khriachtchev, Pettersson, & Runeberg 2000].) Under extreme cold conditions, within a few degrees of absolute zero, clusters of argon atoms with other argon atoms or with some simple gases like HCl or HCN can be made; but they are too weakly held together to be considered normal chemical compounds. Also, argon atoms have been "caged," that is trapped within the structure of other molecules; such "cage compounds" or clathrates are not held together by normal chemical bonds.
It is possible, I suppose, that Berthelot observed such a cage compound [Berthelot 1895]. Benzine is now known as benzene, C6H6, not the complex mixture of light oils from petroleum later called benzine. It is conceivable that an electric discharge may have formed a hydrocarbon cage capable of trapping argon from several ring-shaped benzene molecules. Many years later, Morris Travers offered another explanation. While reading through some correspondence of William Ramsay, whose biography Travers was preparing, he noticed in a letter from Berthelot an expression of thanks for the argon samples Ramsay had sent and a request that he not use iron wire on such samples in the future. Travers, who had worked in Ramsay's lab at the time, knew that copper wire was always used to wrap seals around tubes. He surmised that French customs had unwrapped the wire and replaced it, and in so doing introduced at least some air into the tube, which underwent reaction in Berthelot's experiments.
[44]This sort of problem was also faced by the early "pneumatic chemists" (i.e., the first chemists to distinguish different gases and study them). Some gases they worked with, such as carbon dioxide and ammonia are even more soluble in water than argon. (At 0 °C, the solubility of argon is 2.5 millimoles per liter of water, that of oxygen is 2.2, that of nitrogen is 1.0; for comparison, that of carbon dioxide is 79.) The pneumatic chemists solved their problem by collecting water-soluble ßgases in tubes immersed in mercury rather than water.
[45]When water boils, the steam contains a great deal of heat. Rather than let that heat escape, this device reuses some of the heat contained in steam to warm a new batch of water toward boiling.
[46]Because argon is more easily soluble in water than is nitrogen, the gases dissolved in water exposed to the atmosphere contain a higher proportion of argon (and a lower proportion of nitrogen) than does the atmosphere itself.
[47]In chemistry, spectra are widely used as fingerprints of chemical substances. (Usage note: spectrum is singular; the plural is spectra.) A spectrum was produced by heating a sample until it glowed (in a flame, originally, or by an electric arc) and passing the resulting light through a prism or grating to split it into its components. Typically, the light would be split into a set of characteristic bright lines of distinct wavelengths (which correspond to distinct colors in visible light) [Kirchhoff & Bunsen 1860]. The wavelengths and intensities of these lines constituted a fingerprint of the substance, whose presence in a sample could be deduced by the presence of characteristic lines in a spectrum. This is the kind of spectrum discussed here. Spectroscopic techniques have been extended beyond the realm of visible light to other kinds of electromagnetic radiation, and today relatives of "dark line spectra" (which monitor the wavelengths of radiation absorbed by a sample) are more widespread than the "bright line spectra" mentioned here.
Here is the detailed plate of argon's red and blue spectra, prepared by Crookes and printed in Crookes 1895:
[48]This question illustrates a common attitude of scientists, namely the open-minded appeal to experiment. Here open-minded does not mean that they have no preconception of what an experiment will show; they are open-minded in that they perform experiments even though they have good reason to believe they know what will happen. In other words, they do not simply assume that what they expect to happen will happen. Rayleigh had very good reason to expect that the argon would be the same in both cases, but he put the question to the test (just as he had put the question of nitrogen prepared by two different methods to the test earlier). This time he found what he expected, that argon produced by the two methods was the same as far as his test could tell.
[49]A radiometer has a set of vanes mounted so they can rotate freely within a glass enclosure containing gas at low pressure; it was invented by Crookes. Each vane is black on one side and white on the other. (View a photo of a radiometer from Crookes's time at the Science Museum, London.) When light is shined on the device, the black sides absorb more light (and therefore more energy) than the white. Therefore, the gas molecules which collide with the black sides pick up more energy and recoil with more energy than those which collide with the white. This makes the vanes spin away from the black sides (toward the white sides). The device demonstrates one way in which light energy can be converted into mechanical energy (namely the rotational energy of the vanes). Rayleigh reports that when the gas in the radiometer is argon, the device works the same way is it does with other gases.
[50]Although Rayleigh does not mention it here, this measurement of density allows one to calculate the "molecular weight" (atomic weight, actually) of argon. Because equal volumes of different gases contain equal numbers of molecules, the ratio of the densities of those gases is equal to the ratio of their molecular weights (chapter 9). If argon is 19.9 times as dense as hydrogen, whose molecular weight is known to be 2.02, then argon must have a "molecular weight" of 40.1. The accurate atomic weight of argon is today accepted as 39.948.
[51]The specific heat or heat capacity of a substance is the amount of energy in the form of heat required to raise the temperature of a given amount of that substance by one degree. The heat capacity is different depending on whether the heat is added to a gas kept under a constant pressure, or kept within a fixed volume. In particular, if the volume is not kept constant, some of the heat is used to expand the gas rather than to raise its temperature; under such conditions, then, more heat is required to raise the temperature by one degree than if the volume were kept constant. Thus the constant-volume heat capacity is greater than the constant-pressure heat capacity.
[52]The reasoning here is complex, and depends on some rather arcane results in thermodynamics and in the kinetic theory of gases. The ratio of heat capacities can be no greater than 1.67 (5/3 actually), and that theoretical limit can be reached only by monatomic gases, that is, by gases which exist as single atoms. Without going into the reasons why 5/3 is the ratio for monatomic gases, let us try to understand why the ratio is lower for gases which exist as molecules of two or more atoms (i.e., diatomic or polyatomic molecules) than for monatomic gases. A diatomic molecule such as N2 or O2 has several possible modes of motion: it can fly off as a whole (translational motion), its two atoms can move back and forth in opposite directions (vibration), or it can tumble end over end (rotation). When energy is transferred to such a molecule, that energy can go into any of these modes of motion. By contrast, the only kind of motion available for a monatomic gas is translational motion. Now consider measuring the heat capacity of a gas under conditions of constant pressure. Any heat supplied to the translational motion of a gas will both raise its temperature and make it expand: more vigorous translational motion will make the gas particles collide harder with each other and spread out more if space is available. In contrast, any heat supplied to rotational or vibrational motion will simply raise the temperature of the gas without making it expand. The constant-pressure and constant-volume heat capacities of polyatomic molecules are not all that different, since some of the energy in both cases goes to motion like vibration and rotation which would not expand the gas even when expansion is possible (constant pressure); but the constant-pressure heat capacity of a monatomic gas will be considerably larger than the constant-volume value because all of its energy goes into motion which would expand the gas when expansion is possible.
At present several monatomic gases are known, argon included, and their heat capacity ratios are all 5/3. By contrast, the "ordinary" (diatomic) gases mentioned by Rayleigh have ratios in the vicinity of 1.4, and polyatomic gases such as carbon dioxide (CO2), sulfur dioxide (SO2), and nitrogen dioxide (NO2) have still lower ratios around 1.3.
[53]Rayleigh calls the result awkward because it was new and unexpected: at this time, there were no substances known which were monatomic gases at ordinary temperatures and pressures. The only monatomic gas then known was mercury vapor (that is, not a gas under ordinary conditions). Moreover, if the new gas was monatomic and had a molecular weight of 40, there seemed to be no place for it in the periodic table.
[54]The answers to these questions may seem obvious after the careful description Rayleigh has made of his work up to this time. However, he is summarizing some of the main conclusions of his work and emphasizing the novel and unexpected nature of his results.
[55]Nitrous oxide (N2O) has a molecular weight of 44, which is in the neighborhood of argon's weight. But nitrous oxide has a much lower heat capacity ratio than argon, and it is not inert.
[56]The question of whether the gas is in the atmosphere (and not somehow introduced or formed during the processing of atmospheric air) seems to be answered yes by overwhelming evidence. Yet it takes extremely strong evidence to convince experts on the atmosphere that there was a relatively plentiful component right under their noses (indeed, passing continually into and out of their noses!) without their knowing it.
[57]The technique of "blank" or "control" experiments is frequently a useful one in science. A powerful piece of evidence to support the contention that "X causes Y" is, of course, the observation that after X occurs, Y also happens. But an additional piece of supporting evidence would be to set up a situation in which something like X but not quite the same occurs; if Y does not follow, that is good evidence what causes Y is exactly X or at any rate involves the crucial difference between the two experiments. Here Rayleigh is trying to disprove that his and Ramsay's treatments created or formed argon. He has already shown that argon can be isolated by processing atmospheric air in a certain way; so he now shows that it is not formed by processing nitrogen of non-atmospheric origin ("chemical nitrogen," prepared from urea, for example) in the same way.
[58]Thomas Graham (1805-1869; see portrait photo at National Portrait Gallery, London) was an important early researcher in colloid chemistry and physical chemistry, best known today for his work on the diffusion of gases [Graham 1833].
[59]The point here is that the tobacco pipes change atmospheric air in a way that persists even after oxygen is removed. Rayleigh correctly infers that the change involves the alteration in proportions of nitrogen and a heavier component of the atmosphere, namely argon.
[60]The question of its nature can be put as follows: "Is argon an element, or is it a previously unknown compound of other elements?" Of course, if argon were a compound, the next question would be what is its formula? (I.e., of what elements is it composed?)
[61]The conclusion that argon is an element follows immediately if one accepts that argon is monatomic. And if one accepts that argon is a new substance (i.e., previously unknown), then it follows that it is a new element.
[62]Why was the subject, the question of whether argon was a new element, "difficult" or controversial? Surely part of the difficulty must have been with the accuracy of the measurement of the ratio of specific heats, and of the inference of monatomic behavior from that. Some chemists did not accept the conclusion of monatomicity, which was based on a physical theory (kinetic theory of gases). Furthermore, was 1.65 close enough to 5/3 to be considered practically equal to that theoretical limit? After all, experience with monatomic gases was extremely limited.
But beyond that, there was some resistance to the notion of an unexpected and new element with properties unlike any known element. At first, resistance to the discovery of a new element might seem surprising. After all, the 19th century had seen the discovery and isolation of over 40 new elements already; why not another one? One reason was the periodic table of elements, which had proved extremely useful in organizing the known elements into families of elements with similar properties. Argon had no relatives on the table as it was known then, and there seemed to be no room for it where its atomic weight would put it: the discovery of the new element argon seemed to conflict with an established system of knowledge about the elements--a conflict which disturbed physicists of the time much less than chemists.
For more on the apparent conflict, see Giunta 2001. The conflict did not last long, however; see next chapter for the beginning of the resolution.
[63]Indeed, before there was good evidence that argon was monatomic, the working assumption of the investigators must have been that it was a very stable compound. The previous champion of inertness in the atmosphere, N2, is just that--a very stable and therefore unreactive molecule. It is unreactive because its two atoms are bound to each other very tightly. In order to make N2 react, one must first break apart that strong bond. But what makes argon inert is very different from what makes nitrogen inert: individual argon atoms are not bound to anything, so breaking a strong bond is not a barrier to its forming compounds. This made the inertness of argon even more of a puzzle to its discoverers, for while stable and relatively unreactive compounds were known, free atoms which showed no inclination to combine represented a new phenomenon.
[64]Indeed, N3 as a neutral stable molecule is not known even today, although three nitrogen atoms with an extra electron make a reasonably stable ion (which does not, however, exist under normal conditions as a gas). "Mendeleef" is Dmitri Mendeleev, the man most responsible for developing the periodic system of elements (chapters 12 and 13).
[65]Hermann von Helmholtz (1821-1894; see portrait at Berlin-Brandenburgischen Akademie der Wissenschaften) was a German physicist who also did significant work in physiology and mathematics.
References
References marked with an asterisk (*) may require a subscription to access.
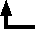 Back to the top of the table of contents of Elements and Atoms.
Back to the top of the table of contents of Elements and Atoms.
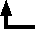 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.