Elements and Atoms: Chapter 13
Mendeleev's later reflections
In 1889 no physical basis for the periodic system of chemical elements was yet known. The periodic table of that day differed in several respects from that of 1869 [Mendeleev 1869] and that of the present day. Yet the periodic system had become widely accepted during the intervening years, during which time several elements predicted by Mendeleev predicted by Mendeleev had been discovered. The Chemical Society had already recognized its importance by awarding the Davy medal to Mendeleev and Julius Lothar Meyer in 1882 and to J. A. R. Newlands in 1887. There had even been time for questions of priority and credit to arise (See, for example, Newlands 1878 and van Spronsen 1969a.), questions which are still debated. The selection reproduced below, then, represents a review of the periodic system by its foremost proponent after its validity and utility had been established but before its foundations had been explored.
The Periodic Law of the Chemical Elements
Professor Mendeléeff, Journal of the Chemical Society (London) 55, 634-656 (1889)
(FARADAY LECTURE delivered before the Fellows of the Chemical Society in the Theatre of the Royal Institution, on Tuesday, June 4th, 1889.)
The high honour bestowed by the Chemical Society in inviting me to pay a tribute to the world-famous name of Faraday by delivering this lecture has induced me to take for its subject the Periodic Law of the Elements--this being a generalisation in chemistry which has of late attracted much attention.
While science is pursuing a steady onward movement[1], it is convenient from time to time to cast a glance back on the route already traversed, and especially to consider the new conceptions which aim at discovering the general meaning of the stock of facts accumulated from day to day in our laboratories. Owing to the possession of laboratories, modern science now bears a new character, quite unknown not only to antiquity but even to the preceding century. Bacon's and Descartes' idea of submitting the mechanism of science simultaneously to experiment and reasoning has been fully realised in the case of chemistry, it having become not only possible but always customary to experiment.[2] Under the all-penetrating control of experiment, a new theory, even if crude, is quickly strengthened, provided it be founded on a sufficient basis; the asperities are removed, it is amended by degrees, and soon loses the phantom light of a shadowy form or of one founded on mere prejudice; it is able to lead to logical conclusions and to submit to experimental proof. Willingly or not, in science we all must submit not to what seems to us attractive from one point of view or from another, but to what represents an agreement between theory and experiment; in other words, to demonstrated generalisation and to the approved experiment. Is it long since many refused to accept the generalisations involved in the law of Avogadro and Ampère, so widely extended by Gerhardt?[3] We still may hear the voices of its opponents; they enjoy perfect freedom, but vainly will their voices rise so long as they do not use the language of demonstrated facts. The striking observations with the spectroscope which have permitted us to analyse the chemical constitution of distant worlds, seemed, at first, applicable to the task of determining the nature of the atoms themselves; but the working out of the idea in the laboratory soon demonstrated that the characters of spectra are determined--not directly by the atoms, but by the molecules into which the atoms are packed; and so it became evident that more verified facts must be collected before it will be possible to formulate new generalisations capable of taking their place beside those ordinary ones based upon the conception of simple bodies and atoms.[4] But as the shade of the leaves and roots of living plants, together with the relics of a decayed vegetation, favour the growth of the seedling and serve to promote its luxurious development, in like manner sound generalisations--together with the relics of those which have proved to be untenable--promote scientific productivity, and ensure the luxurious growth of science under the influence of rays emanating from the centres of scientific energy.[5] Such centres are scientific associations and societies. Before one of the oldest and most powerful of these I am about to take the liberty of passing in review the 20 years' life of a generalisation which is known under the name of the Periodic Law. It was in March, 1869, that I ventured to lay before the then youthful Russian Chemical Society the ideas upon the same subject, which I had expressed in my just written "Principles of Chemistry."[6]
Without entering into details, I will give the conclusions I then arrived at, in the very words I used:--[7]
"1. The elements, if arranged according to their atomic weights, exhibit an evident periodicity of properties.
"2. Elements which are similar as regards their chemical properties have atomic weights which are either of nearly the same value (e.g., platinum, iridium, osmium) or which increase regularly (e.g., potassium, rubidium, caesium).
"3. The arrangement of the elements, or of groups of elements in the order of their atomic weights corresponds to their so-called valencies as well as, to some extent, to their distinctive chemical properties--as is apparent among other series in that of lithium, beryllium, barium [sic: boron is meant--CJG], carbon, nitrogen, oxygen and iron [sic: fluorine is meant--CJG].[8]
"4. The elements which are the most widely diffused have small atomic weights.
"5. The magnitude of the atomic weight determines the character of the element just as the magnitude of the molecule determines the character of a compound body.
"6. We must expect the discovery of many yet unknown elements, for example, elements analogous to aluminium and silicon, whose atomic weight would be between 65 and 75.
"7. The atomic weight of an element may sometimes be amended by a knowledge of those of the contiguous elements. Thus, the atomic weight of tellurium must lie between 123 and 126, and cannot be 128.
"8. Certain characteristic properties of the elements can be foretold from their atomic weights.
"The aim of this communication will be fully attained if I succeed in drawing the attention of investigators to those relations which exist between the atomic weights of dissimilar elements, which, as far as I know, have hitherto been almost completely neglected. I believe that the solution of some of the most important problems of our science lies in researches of this kind."
To-day, 20 years after the above conclusions were formulated, they may still be considered as expressing the essence of the now well-known periodic law.
Reverting to the epoch terminating with the sixties, it is proper to indicate three series of data without the knowledge of which the periodic law could not have been discovered, and which rendered its appearance natural and intelligible.
In the first place, it was at that time that the numerical value of atomic weights became definitely known. Ten years earlier such knowledge did not exist, as may be gathered from the fact that in 1860 chemists from all parts of the world met at Karlsruhe in order to come to some agreement, if not with respect to views relating to atoms, at any rate as regards their definite representation. Many of those present probably remember how vain were the hopes of coming to an understanding, and how much ground was gained at that Congress by the followers of the unitary theory so brilliantly represented by Cannizzaro.[9] I vividly remember the impression produced by his speeches, which admitted of no compromise, and seemed to advocate truth itself, based on the conceptions of Avogadro, Gerhardt and Regnault, which at that time were far from being generally recognised. And though no understanding could be arrived at, yet the objects of the meeting were attained, for the ideas of Cannizzaro proved, after a few years, to be the only ones which could stand criticism, and which represented an atom as--"the smallest portion of an element which enters into a molecule of its compound." Only such real atomic weights--not conventional ones[10]--could afford a basis for generalisation. It is sufficient, by way of example, to indicate the following cases in which the relation is seen at once and is perfectly clear:--
| K = 39 | Rb = 85 | Cs = 133
|
| Ca = 40 | Sr = 87 | Ba = 137 |
whereas with the equivalents then in use--
| K = 39 | Rb = 85 | Cs = 133
|
| Ca = 20 | Sr = 43.5 | Ba = 68.5 |
the consecutiveness of change in atomic weight, which with the true values is so evident, completely disappears.
Secondly, it had become evident during the period 1860-70, and even during the preceding decade, that the relations between the atomic weights of analogous elements were governed by some general and simple laws. Cooke, Cremers, Gladstone, Gmelin, Lenssen, Pettenkofer, and especially Dumas, had already established many facts bearing on that view. Thus Dumas compared the following groups of analogous elements with organic radicles[11]--
| Diff. | | Diff. | | Diff. | | Diff.
|
|---|
| | Mg = 12 | | P = 31 | | O = 8 |
|
| | | 8 | | 44 | | 8
|
| Li = 7 | | Ca = 20 | | As = 75 | | S = 16 |
|
| 16 | | 3 x 8 | | 44 | | 3 x 8
|
| Na = 23 | | Sr = 44 | | Sb = 119 | | Se = 40 |
|
| 16 | | 3 x 8 | | 2 x 44 | | 3 x 8
|
| K = 39 | | Ba = 68 | | Bi = 207 | | Te = 64 |
and pointed out some really striking relationships, such as the following:--
F = 19.
Cl = 35.5 = 19 + 16.5.
Br = 80 = 19 + 2 x 16.5 + 28.
I = 127 = 2 x 19 + 2 x 16.5 + 2 x 28.
A. Strecker, in his work "Theorien und Experimente zur Bestimmung der Atomgewichte der Elemente" (Braunschweig, 1859), after summarising the data relating to the subject, and pointing out the remarkable series of equivalents--
Cr = 26.2 Mn = 27.6 Fe = 28 Ni = 29 Co = 30
Cu = 31.7 Zn = 32.5
remarks that: "It is hardly probable that all the above-mentioned relations between the atomic weights (or equivalents) of chemically analogous elements are merely accidental. We must, however, leave to the future the discovery of the law of the relations which appears in these figures."[12]
In such attempts at arrangement and in such views are to be recognised the real forerunners of the periodic law; the ground was prepared for it between 1860 and 1870, and that it was not expressed in a determinate form before the end of the decade, may, I suppose, be ascribed to the fact that only analogous elements had been compared. The idea of seeking for a relation between the atomic weights of all the elements was foreign to the ideas then current, so that neither the vis tellurique of De Chancourtois[13], nor the law of octaves of Newlands[14], could secure anybody's attention. And yet both De Chancourtois and Newlands, like Dumas and Strecker, more than Lenssen and Pettenkofer, had made an approach to the periodic law and had discovered its germs. The solution of the problem advanced but slowly, because the facts, and not the law, stood foremost in all attempts; and the law could not awaken a general interest so long as elements, having no apparent connection with each other, were included in the same octave, as for example:--
| 1st octave of Newlands[15] | H | F | Cl | Co & Ni | Br | Pd | I | Pt & Ir
|
|---|
| 7th Ditto | O | S | Fe | Se | Rh & Ru | Te | Au | Os or Th |
|---|
Analogies of the above order seemed quite accidental, and the more so as the octave contained occasionally 10 elements instead of eight, and when two such elements as Ba and V, Co and Ni, or Rh and Ru, occupied one place in the octave.[16] Nevertheless, the fruit was ripening, and I now see clearly that Strecker, De Chancourtois and Newlands stood foremost in the way toward the discovery of the periodic law, and that they merely wanted the boldness necessary to place the whole question at such a height that its reflection on the facts could be clearly seen.
A third circumstance which revealed the periodicity of chemical elements was the accumulation, by the end of the sixties, of new information respecting the rare elements, disclosing their many-sided relations to the other elements and to each other. The researches of Marignac on niobium, and those of Roscoe on vanadium were of special moment. The striking analogies between vanadium and phosphorus on the one hand, and between vanadium and chromium on the other, which became so apparent in the investigations connected with that element, naturally induced the comparison of V = 51 with Cr = 52, Nb = 94 with Mo = 96, and Ta = 192 with W = 194; while, on the other hand, P = 31 could be compared with S = 32, As = 75 with Se = 79, and Sb = 120 with Te = 125. From such approximations there remained but one step to the discovery of the law of periodicity.[17]
The law of periodicity was thus a direct outcome of the stock of generalisations and established facts which had accumulated by the end of the decade 1860-1870: it is an embodiment of those data in a more or less systematic expression.[18] Where, then, lies the secret of the special importance which has since been attached to the periodic law, and has raised it to the position of a generalisation which has already given to chemistry unexpected aid, and which promises to be far more fruitful in the future and to impress upon several branches of chemical research a peculiar and original stamp? The remaining part of my communication will be an attempt to answer this question.
In the first place we have the circumstance that, as soon as the law, made its appearance, it demanded a revision of many facts which were considered by chemists as fully established by existing experience. I shall return, later on, briefly to this subject, but I wish now to remind you that the periodic law, by insisting on the necessity for a revision of supposed facts, exposed itself at once to destruction in its very origin.[19] Its first requirements, however, have been almost entirely satisfied during the last 20 years; the supposed facts have yielded to the law, thus proving that the law itself was a legitimate induction from the verified facts. But our inductions from data have often to do with such details of a science so rich in facts, that only generalisations which cover a wide range of important phenomena can attract general attention. What were the regions touched on by the periodic law? This is what we shall now consider.
The most important point to notice is, that periodic functions, used for the purpose of expressing changes which are dependent on variations of time and space, have been long known. They are familiar to the mind when we have to deal with motion in closed cycles, or with any kind of deviation from a stable position, such as occurs in pendulum-oscillations.[20] A like periodic function became evident in the case of the elements, depending on the mass of the atom. The primary conception of the masses of bodies or of the masses of atoms belongs to a category which the present state of science forbids us to discuss, because as yet we have no means of dissecting or analysing their conception. All that was known of functions dependent on masses derived its origin from Galileo and Newton, and indicated that such functions either decrease or increase with the increase of mass, like the attraction of celestial bodies. The numerical expression of the phenomena was always found to be proportional to the mass, and in no case was an increase of mass followed by a recurrence of properties such as is disclosed by the periodic law of the elements.[21] This constituted such a novelty in the study of the phenomena of nature that, although it did not lift the veil which conceals the true conception of mass, it nevertheless indicated that the explanation of that conception must be searched for in the masses of the atoms; the more so, as all masses are nothing but aggregations, or additions, of chemical atoms which would be best described as chemical individuals. Let me remark by the way that though the Latin word "individual" is merely a translation of the Greek word "atom," nevertheless history and custom have drawn so sharp a distinction between the two words, and the present chemical conception of atoms is nearer to that defined by the Latin word than by the Greek, although this latter also has acquired a special meaning which was unknown to the classics.[22] The periodic law has shown that our chemical individuals display a harmonic periodicity of properties, dependent on their masses. Now, natural science has long been accustomed to deal with periodicities observed in nature, to seize them with the vice of mathematical analysis, to submit them to the rasp of experiment. And these instruments of scientific thought would surely, long since, have mastered the problem connected with the chemical elements, were it not for a new feature which was brought to light by the periodic law and which gave a peculiar and original character to the periodic function.
If we mark on an axis of abscissae a series of lengths proportional to angles, and trace ordinates which are proportional to sines or other trigonometrical functions, we get periodic curves of a harmonic character.[23] So it might seem, at first sight, that with the increase of atomic weights the function of the properties of the elements should also vary in the same harmonious way. But in this case there is no such continuous change as in the curves just referred to, because the periods do not contain the infinite number of points constituting a curve, but a finite number only of such points.[24] An example will better illustrate this view. The atomic weights--
| Ag = 108 | Cd = 112 | In = 113 | Sn = 118
|
| Sb = 120 | Te = 125 | I = 127 |
steadily increase, and their increase is accompanied by a modification of many properties which constitutes the essence of the periodic law. Thus, for example, the densities of the above elements decrease steadily, being respectively--
while their oxides contain an increasing quantity of oxygen:--
| Ag2O | Cd2O2 | In2O3 | Sn2O4 | Sb2O5 | Te2O6 | I2O7 |
But to connect by a curve the summits of the ordinates expressing any of these properties would involve the rejection of Dalton's law of multiple proportions. Not only are there no intermediate elements between silver, which gives AgCl, and cadmium, which gives CdCl2, but, according to the very essence of the periodic law there can be none; in fact a uniform curve would be inapplicable in such a case, as it would lead us to expect elements possessed of special properties at any point of the curve.[25] The periods of the elements have thus a character very different from those which are so simply represented by geometers. They correspond to points, to numbers, to sudden changes of the masses, and not to a continuous evolution. In these sudden changes destitute of intermediate steps or positions, in the absence of elements intermediate between, say, silver and cadmium, or aluminium and silicon, we must recognise a problem to which no direct application of the analysis of the infinitely small can be made. Therefore, neither the trigonometrical functions proposed by Ridberg and Flavitzky, nor the pendulum-oscillations suggested by Crookes, nor the cubical curves of the Rev. Mr. Haughton, which have been proposed for expressing the periodic law, from the nature of the case, can represent the periods of the chemical elements. If geometrical analysis is to be applied to this subject it will require to be modified in a special manner. It must find the means of representing in a special way not only such long periods as that comprising,
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga G[e] As Se Br,
but short periods like the following:--
Na Mg Al Si P S Cl.[26]
In the theory of numbers[27] only do we find problems analogous to ours, and two attempts at expressing the atomic weights of the elements by algebraic formulae seem to be deserving of attention, although neither of them can be considered as a complete theory, nor as promising finally to solve the problem of the periodic law. The attempt of E. J. Mills (1886) does not even aspire to attain this end. He considers that all atomic weights can be expressed by a logarithmic function,
15(n - 0.9375t),
in which the variables n and t are whole numbers. Thus, for oxygen, n = 2, and t = 1, whence its atomic weight is = 15.94; in the case of chlorine, bromine, and iodine, n has respective values of 3, 6, and 9, while t = 14, 18, and 20.
Another attempt was made in 1888 by B. N. Tchitchérin. Its author places the problem of the periodic law in the first rank, but as yet he has investigated the alkaline metals only. Tchitchérin first noticed the simple relations existing between the atomic volumes of all alkaline metals; they can be expressed, according to his views, by the formula
A(2 - 0.00535An),
where A is the atomic weight, and n is equal to 8 for lithium and sodium, to 4 for potassium, to 3 for rubidium, and to 2 for caesium. If n remained equal to 8, during the increase of A, then the volume would become zero at A = 462/3, and it would reach its maximum at A = 231/3. The close approximation of the number 462/3 to the difference between the atomic weights of analogous elements (such as Cs - Rb, I - Br, and so on); the close correspondence of the number 231/3 to the atomic weight of sodium; the fact of n being necessarily a whole number, and several other aspects of the question, induce Tchitchérin to believe that they afford a clue to the understanding of the nature of the elements; we must, however, await the full development of his theory before pronouncing judgment on it.[28] What we can at present only be certain of is this: that attempts like the two above named must be repeated and multiplied, because the periodic law has clearly shown that the masses of the atoms increase abruptly, by steps, which are clearly connected in some way with Dalton's law of multiple proportions; and because the periodicity of the elements finds expression in the transition from RX to RX2, RX3, RX4, and so on till RX8, at which point the energy of the combining forces being exhausted, the series begins anew from RX to RX2, and so on.[29]
While connecting by new bonds the theory of the chemical elements with Dalton's theory of multiple proportions, or atomic structure of bodies, the periodic law opened for natural philosophy a new and wide field for speculation. Kant said that there are in the world "two things which never cease to call for the admiration and reverence of man: the moral law within ourselves, and the stellar sky above us." But when we turn our thoughts towards the nature of the elements and the periodic law, we must add a third subject, namely, "the nature of the elementary individuals which we discover everywhere around us." Without them the stellar sky itself is inconceivable; and in the atoms we see at once their peculiar individualities, the infinite multiplicity of the individuals, and the submission of their seeming freedom to the general harmony of Nature.
Having thus indicated a new mystery of Nature, which does not yet yield to rational conception, the periodic law, together with the revelations of spectrum analysis, have contributed to again revive an old but remarkably long-lived hope--that of discovering, if not by experiment, at least, by a mental effort, the primary matter[30]--which had its genesis in the minds of the Grecian philosophers, and has been transmitted, together with many other ideas of the classic period, to the heirs of their civilisation. Having grown, during the times of the alchemists up to the period when experimental proof was required, the idea has rendered good service; it induced those careful observations and experiments which later on called into being the works of Scheele, Lavoisier, Priestley and Cavendish. It then slumbered awhile, but was soon awakened by the attempts either to confirm or to refute the ideas of Prout as to the multiple proportion relationship of the atomic weights of all the elements. And once again the inductive or experimental method of studying Nature gained a direct advantage from the old Pythagorean idea[31]: because atomic weights were determined with an accuracy formerly unknown. But again the idea could not stand the ordeal of experimental test, yet the prejudice remains and has not been uprooted, even by Stas[32]; nay, it has gained a new vigour, for we see that all which is imperfectly worked out, new and unexplained, from the still scarcely studied rare metals to the hardly perceptible nebulae, have been used to justify it. As soon as spectrum analysis appears as a new and powerful weapon of chemistry, the idea of a primary matter is immediately attached to it. From all sides we see attempts to constitute the imaginary substance helium[33] the so much longed for primary matter.[34] No attention is paid to the circumstance that the helium line is only seen in the spectrum of the solar protuberances, so that its universality in Nature remains as problematic as the primary matter itself; nor to the fact that the helium line is wanting amongst the Fraunhofer lines of the solar spectrum, and thus does not answer to the brilliant fundamental conception which gives its real force to spectrum analysis.
And finally, no notice is even taken of the indubitable fact that the brilliancies of the spectral lines of the simple bodies vary under different temperatures and pressures; so that all probabilities are in favour of the helium line simply belonging to some long since known element placed under such conditions of temperature, pressure, and gravity as have not yet been realised in our experiments. Again, the idea that the excellent investigations of Lockyer of the spectrum of iron can be interpreted in favour of the compound nature of that element, evidently must have arisen from some misunderstanding. The spectrum of a compound body certainly does not appear as a sum of the spectra of its components; and therefore the observations of Lockyer can be considered precisely as a proof that iron undergoes no other changes at the temperature of the sun but those which it experiences in the voltaic arc--provided the spectrum of iron is preserved. As to the shifting of some of the lines of the spectrum of iron while the other lines maintain their positions, it can be explained, as shown by M. Kleiber (Journal of the Russian Chemical and Physical Society, 1885, 147), by the relative motion of the various strata of the sun's atmosphere, and by Zöllner's laws of the relative brilliancies of different lines of the spectrum. Moreover, it ought not to be forgotten that if iron were really proved to consist of two or more unknown elements, we simply should have an increase of the number of our elements--not a reduction, and still less a reduction of all of them to one single primary matter.
Feeling that spectrum analysis will not yield a support to the Pythagorean conception, its modern promoters are so bent upon its being confirmed by the periodic law, that the illustrious Berthelot, in his work Les origines de l'Alchimie, 1885, 313, has simply mixed up the fundamental idea of the law of periodicity with the ideas of Prout, the alchemists, and Democritus about primary matter.[35] But the periodic law, based as it is on the solid and wholesome ground of experimental research, has been evolved independently of any conception as to the nature of the elements;[36] it does not in the least originate in the idea of an unique matter; and it has no historical connection with that relic of the torments of classical thought, and therefore it affords no more indication of the unity of matter or of the compound character of our elements, than the law of Avogadro, or the law of specific heats,[37] or even the conclusions of spectrum analysis. None of the advocates of an unique matter have ever tried to explain the law from the standpoint of ideas taken from a remote antiquity when it was found convenient to admit the existence of many gods--and of an unique matter.
When we try to explain the origin of the idea of an unique primary matter, we easily trace that in the absence of inductions from experiment it derives its origin from the scientifically philosophical attempt at discovering some kind of unity in the immense diversity of individualities which we see around. In classical times such a tendency could only be satisfied by conceptions about the immaterial world. As to the material world, our ancestors were compelled to resort to some hypothesis, and they adopted the idea of unity in the formative material, because they were not able to evolve the conception of any other possible unity in order to connect the multifarious relations of matter. Responding to the same legitimate scientific tendency, natural science has discovered throughout the universe a unity of plan, a unity of forces, and a unity of matter, and the convincing conclusions of modern science compel everyone to admit these kinds of unity. But while we admit unity in many things, we none the less must also explain the individuality and the apparent diversity which we cannot fail to trace everywhere. It has been said of old, "Give a fulcrum, and it will become easy to displace the earth." So also we must say, "Give anything that is individualised, and the apparent diversity will be easily understood." Otherwise, how could unity result in a multitude?
After a long and painstaking research, natural science has discovered the individualities of the chemical elements, and therefore it is now capable not only of analysing, but also of synthesising; it can understand and grasp the general and unity, as well as the individualised and the multitudinous. Unity and the general, like time and space, like force and motion, vary uniformly; the uniform admit of interpolations, revealing every intermediate phase. But the multitudinous, the individualised--like ourselves, like the chemical elements, like the members of a peculiar periodic function of elements, like Dalton's multiple proportions--is characterised in another way: we see in it--side by side with a connecting general principle--leaps, breaks of continuity, points which escape from the analysis of the infinitely small--a complete absence of intermediate links.[38] Chemistry has found an answer to the question as to the causes of multitudes; and while retaining the conception of many elements, all submitted to the discipline of a general law, it offers an escape from the Indian Nirvana--the absorption in the universal, replacing it by the individualised. However, the place for individuality is so limited by the all-grasping, all-powerful universal, that it is merely a fulcrum for the understanding of multitude in unity.
Having touched upon the metaphysical bases of the conception of an unique matter which is supposed to enter into the composition of all bodies, I think it necessary to dwell upon another theory, akin to the above conception,--the theory of the compound character of the elements now admitted by some,--and especially upon one particular circumstance which being related to the periodic law is considered to be an argument in favour of that hypothesis.
Dr. Pelopidas, in 1883, made a communication to the Russian Chemical and Physical Society on the periodicity of the hydrocarbon radicles, pointing out the remarkable parallelism which was to be noticed in the change of properties of hydrocarbon radicles and elements when classed in groups. Professor Carnelley, in 1886, developed a similar parallelism. The idea of M. Pelopidas will be easily understood if we consider the series of hydrocarbon radicles which contain, say, 6 atoms of carbon:--
| I | II | III | IV | V | VI | VII | VIII
|
| C6H13 | C6H12 | C6H11 | C6H10 | C6H9 | C6H8 | C6H7 | C6H6 |
The first of these radicles, like the elements of the Ist group, combines with Cl, OH, and so on, and gives the derivatives of hexyl alcohol, C6H13(OH); but, in proportion as the number of hydrogen atoms decreases, the capacity of the radicles of combining with, say, the halogens increases. C6H12 already combines with 2 atoms of chlorine; C6H11 with 3 atoms, and so on. The last members of the series comprise the radicles of acids; thus C6H8, which belongs to the VIth group, gives, like sulphur, a bibasic acid, C6H8O2(OH)2, which is homologous with oxalic acid. The parallelism can be traced still further--because C6H5 appears as a monovalent radicle of benzene--and with it begins a new series of aromatic derivatives, so analogous to the derivatives of the fat series. Let me also mention another example from among those which have been given by M. Pelopidas. Starting from the alkaline radicle of monomethylammonium, N(CH3)H3, or NCH6, which presents many analogies with the alkaline metals of the Ist group, he arrives, by successively diminishing the number of the atoms of hydrogen, at a seventh group which contains cyanogen, CN, which has long since been compared to the halogens of the VIIth group.
The most important consequence which, in my opinion, can be drawn from the above comparison is that the periodic law, so apparent in the elements, has a wider application than might appear at first sight; it opens up a new vista of chemical evolutions. But, while admitting the fullest parallelism between the periodicity of the elements and that of the compound radicles, we must not forget that in the periods of the hydrocarbon radicles we have a decrease of mass as we pass from the representatives of the first group to the next; while in the periods of the elements the mass increases during the progression. It thus becomes evident that we cannot speak of an identity of periodicity in both cases, unless we put aside the ideas of mass and attraction, which are the real corner-stones of the whole of natural science and even enter into those very conceptions of simple bodies which came to light a full hundred years later than the immortal principles of Newton.[39],[40]
From the foregoing, as well as from the failures of so many attempts at finding in experiment and speculation a proof of the compound character of the elements and of the existence of primordial matter, it is evident, in my opinion, that this theory must be classed amongst mere utopias. But utopias can only be combatted by freedom of opinion, by experiment, and by new utopias. In the republic of scientific theories freedom of opinions is guaranteed. It is precisely that freedom which permits me to criticise openly the widely diffused idea as to the unity of matter in the elements. Experiments and attempts at confirming that idea have been so numerous that it really would be instructive to have them all collected together, if only to serve as a warning against the repetition of old failures. And, now, as to new utopias which may be helpful in the struggle against the old ones, I do not think it quite useless to mention a phantasy of one of my students who imagined that the weight of bodies does not depend upon their mass, but upon the character of the motion of their atoms. The atoms, according to this new utopian, may all be homogeneous or heterogeneous, we know not which; we know them in motion only, and that motion they maintain with the same persistence as the stellar bodies maintain theirs. The weights of atoms differ only in consequence of their various modes and quantity of motion; the heaviest atoms may be much simpler than the lighter ones; thus an atom of mercury may be simpler than an atom of hydrogen--the manner in which it moves causes it to be heavier. My interlocutor even suggested that the view which attributes the greater complexity to the lighter elements finds confirmation in the fact that the hydrocarbon radicles mentioned by Pelopidas, while becoming lighter as they lose hydrogen, change their properties periodically in the same manner as the elements change theirs according as the atoms grow heavier.
The French proverb, La critique est facile mais l'art est difficile, however, may well be reversed in the case of all such ideal views, as it is much easier to formulate than to criticize them. Arising from the virgin soil of newly established facts, the knowledge relating to the elements, to their masses, and to the periodic changes of their properties, has given a motive for the formation of utopian hypotheses, probably because they could not be foreseen by the aid of any of the various metaphysical systems, and exist, like the idea of gravitation, as an independent outcome of natural science, requiring the acknowledgment of general laws, when these have been established with the same degree of persistency as is indispensable for the acceptance of a thoroughly established fact. Two centuries have elapsed since the theory of gravitation was enunciated, and although we do not understand its cause, we still must regard gravitation as a fundamental conception of natural philosophy, a conception which has enabled us to perceive much more than the metaphysicians did or could with their seeming omniscience. A hundred years later the conception of the elements arose;[41] it made chemistry what it now is; and yet we have advanced as little in our comprehension of simple bodies since the times of Lavoisier and Dalton as we have in our understanding of gravitation. The periodic law of the elements is only 20 years old: it is not surprising therefore that, knowing nothing about the causes of gravitation and mass, or about the nature of the elements, we do not comprehend the rationale of the periodic law. It is only by collecting established laws, that is by working at the acquirement of truth, that we can hope gradually to lift the veil which conceals from us the causes of the mysteries of Nature and to discover their mutual dependency. Like the telescope and the microscope, laws founded on the basis of experiment are the instruments and means of enlarging our mental horizon.[42]
In the remaining part of my communication I shall endeavour to show, and as briefly as possible, in how far the periodic law contributes to enlarge our range of vision. Before the promulgation of this law the chemical elements were mere fragmentary, incidental facts in Nature; there was no special reason to expect the discovery of new elements, and the new ones which were discovered from time to time appeared to be possessed of quite novel properties. The law of periodicity first enabled us to perceive undiscovered elements at a distance which formerly was inaccessible to chemical vision; and long ere they were discovered new elements appeared before our eyes possessed of a number of well-defined properties. We now know three cases of elements whose existence and properties were foreseen by the instrumentality of the periodic law. I need but mention the brilliant discovery of gallium, which proved to correspond to eka-aluminium of the periodic law, by Lecoq de Boisbaudran; of scandium, corresponding to eka-boron, by Nilson; and of germanium, which proved to correspond in all respects to eka-silicium, by Winckler. When, in 1871, I described to the Russian Chemical Society the properties, clearly defined by the periodic law, which such elements ought to possess, I never hoped that I should live to mention their discovery to the Chemical Society of Great Britain as a confirmation of the exactitude and the generality of the periodic law. Now, that I have had the happiness of doing so, I unhesitatingly say that although greatly enlarging our vision, even now the periodic law needs further improvements in order that it may become a trustworthy instrument in further discoveries.[43],[44]
I will venture to allude to some other matters which chemistry has discerned by means of its new instrument, and which it could not have made out without a knowledge of the law of periodicity, and I will confine myself to simple bodies and to oxides.
Before the periodic law was formulated the atomic weights of the elements were purely empirical numbers, so that the magnitude of the equivalent, and the atomicity or the value in substitution possessed by an atom, could only be tested by critically examining the methods of determination, but never directly by considering the numerical values themselves; in short, we were compelled to move in the dark, to submit to the facts, instead of being masters of them. I need not recount the methods which permitted the periodic law at last to master the facts relating to atomic weights, and I would merely call to mind that it compelled us to modify the valencies of indium and cerium, and to assign to their compounds a different molecular composition. Determinations of the specific heats of these two metals fully confirmed the change. The trivalency of yttrium, which makes us now represent its oxide as Y2O3 instead of as YO, was foreseen (in 1870) by the periodic law, and it now has become so probable that Cleve, and all other subsequent investigators of the rare metals, have not only adopted it but have also applied it without any new demonstration to bodies so imperfectly known as those of the cerite and gadolinite group, especially since Hildebrand determined the specific heats of lanthanum and didymium and confirmed the expectations suggested by the periodic law. But here, especially in the case of didymium, we meet with a series of difficulties long since foreseen through the periodic law, but only now becoming evident, and chiefly arising from the relative rarity and insufficient knowledge of the elements which usually accompany didymium.[45]
Passing to the results obtained in the case of the rare elements beryllium, scandium and thorium, it is found that these have many points of contact with periodic law. Although Avdéeff long since proposed the magnesia formula to represent beryllium oxide, yet there was so much to be said in favour of the alumina formula, on account of the specific heat of the metals and the isomorphism of the two oxides, that it became generally adopted and seemed to be well established. The periodic law, however, as Brauner repeatedly insisted (Berichte, 1878, 872; 1881, 53) was against the formula Be2O3; it required the magnesium formula BeO, that is, an atomic weight of 9, because there was no place in the system for an element like beryllium having an atomic weight of 13.5. This divergence of opinion lasted for years, and I often heard that the question as to the atomic weight of beryllium threatened to disturb the generality of the periodic law, or, at any rate, to require some important modifications of it. Many forces were operating in the controversy regarding beryllium, evidently because a much more important question was at issue than merely that involved in the discussion of the atomic weight of a relatively rare element; and during the controversy the periodic law became better understood, and the mutual relations of the elements became more apparent than ever before. It is most remarkable that the victory of the periodic law was won by the researches of the very observers who previously had discovered a number of facts in support of the trivalency of beryllium. Applying the higher law of Avogadro, Nilson and Petterson have finally shown that the density of the vapour of the beryllium chloride, BeCl2, obliges us to regard beryllium as bivalent in conformity with the periodic law.[46] I consider the confirmation of Avdéeff's and Brauner's view as important in the history of the periodic law as the discovery of scandium, which, in Nilson's hands, confirmed the existence of the eka-boron.[47]
The circumstance that thorium proved to be quadrivalent, and Th = 232, in accordance with the views of Chydenius and the requirements of the periodic law, passed almost unnoticed, and was accepted without opposition, and yet both thorium and uranium are of great importance in the periodic system, as they are its last members and have the highest atomic weights of all the highest elements.
The alteration of the atomic weight of uranium from U = 120 into U = 240 attracted more attention, the change having been made on account of the periodic law, and for no other reason. Now that Roscoe, Rammelsberg, Zimmermann, and several others have admitted the various claims of the periodic law in the case of uranium, its high atomic weight is received without objection, and it endows that element with a special interest.[48]
While thus demonstrating the necessity of modifying the atomic weights of several insufficiently known elements, the periodic law enabled us also to detect errors in the determination of the atomic weights of several elements whose valencies and true position among other elements were already well known. Three such cases are especially noteworthy: those of tellurium, titanium and platinum. Berzelius had determined the atomic weight of tellurium to be 128, while the periodic law claimed for it an atomic weight below that of iodine, which had been fixed by Stas at 126.5, and which was certainly not higher than 127. Brauner then undertook the investigation, and he has shown that the true atomic weight of tellurium is lower than that of iodine, being near to 125.[49] For titanium the extensive researches of Thorpe have confirmed the atomic weight of Ti = 48, indicated by the law, and already foreseen by Rose, but contradicted by the analyses of Pierre and several other chemists. An equally brilliant confirmation of the expectations based on the periodic law has been given in the case of the series osmium, iridium, platinum, and gold. At the time of the promulgation of the periodic law the determinations of Berzelius, Rose, and many others gave the following figures:--
Os = 200; Ir = 197; Pt = 198; Au = 196.
The expectations of the periodic law[50] have been confirmed[51], first, by new determinations of the atomic weight of platinum (by Seubert, Dittmar and M'Arthur), which proved to be near to 196 (taking O = 16, as proposed by Marignac, Brauner, and others); secondly, by Seubert having proved that the atomic weight of osmium is really lower than that of platinum, and that it is near to 191; and thirdly, by the investigations of Krüss, and Thorpe and Laurie proving that the atomic weight of gold exceeds that of platinum, and approximates to 197. The atomic weights which were thus found to require correction were precisely those which the periodic law had indicated as affected with errors; and it has been proved therefore that the periodic law affords a means of testing experimental results. If we succeed in discovering the exact character of the periodical relationships between the increments in atomic weights of allied elements discussed by Ridberg in 1885, and again by Razaroff in 1887, we may expect that our instrument will give us the means of still more closely controlling the experimental data relating to atomic weights.
Let me next call to mind that, while disclosing the variation of chemical properties,[52] the periodic law has also enabled us to systematically discuss many of the physical properties of elementary bodies, and to show that these properties are also subject to the law of periodicity. At the Moscow Congress of Russian Naturalists in August, 1869, I dwelt upon the relations which existed between density and the atomic weight of the elements. The following year Professor Lothar Meyer, in his well-known paper,[53] studied the same subject in more detail, and thus contributed to spread information about the periodic law. Later on, Carnelley, Laurie, L. Meyer, Roberts-Austen, and several others applied the periodic system to represent the order in the changes of the magnetic properties of the elements, their melting points, the heats of formation of their haloid compounds, and even of such mechanical properties as the coefficient of elasticity, the breaking stress, &c., &c. These deductions, which have received further support in the discovery of new elements endowed not only with chemical but even with physical properties which were foreseen by the law of periodicity, are well known; so I need not dwell upon the subject, and may pass to the consideration of oxides.[54]
In indicating that the gradual increase of the power of elements of combining with oxygen is accompanied by a corresponding decrease in their power of combining with hydrogen, the periodic law has shown that there is a limit of oxidation, just as there is a well-known limit to the capacity of elements for combining with hydrogen. A single atom of an element combines with at most four atoms of either hydrogen or oxygen: and while CH4 and SiH4 represent the highest hydrides, so RuO4 and OsO4 are the highest oxides. We are thus led to recognise types of oxides, just as we have had to recognise types of hydrides.[55]
The periodic law has demonstrated that the maximum extent to which different non-metals enter into combination with oxygen is determined by the extent to which they combine with hydrogen, and that the sum of the number of equivalents of both must be equal to 8. Thus chlorine, which combines with 1 atom, or 1 equivalent of hydrogen, cannot fix more than 7 equivalents of oxygen, giving Cl2O7: while sulphur, which fixes 2 equivalents of hydrogen, cannot combine with more than 6 equivalents or 3 atoms of oxygen. It thus becomes evident that we cannot recognise as a fundamental property of the elements the atomic valencies deduced from their hydrides; and that we must modify, to a certain extent, the theory of atomicity if we desire to raise it to the dignity of a general principle capable of affording an insight into the constitution of all compound molecules. In other words, it is only to carbon, which is quadrivalent with regard both to oxygen and hydrogen, that we can apply the theory of constant valency and of bond, by means of which so many still endeavour to explain the structure of compound molecules. But I should go too far if I ventured to explain in detail the conclusions which can be drawn from the above considerations. Still, I think it necessary to dwell upon one particular fact which must be explained from the point of view of the periodic law in order to clear the way to its extension in that particular direction.
The higher oxides yielding salts the formation of which was foreseen by the periodic system--for instance, in the short series beginning with sodium--
Na2O, MgO, Al2O3, SiO2, P2O5, SO3, Cl2O7,
must be clearly distinguished from the higher degrees of oxidation which correspond to hydrogen peroxide and bear the true character of peroxides. Peroxides such as Na2O2, BaO2, and the like have long been known. Similar peroxides have also recently become known in the case of chromium, sulphur, titanium, and many other elements, and I have sometimes heard it said that discoveries of this kind weaken the conclusions of the periodic law in so far as it concerns the oxides. I do not think so in the least, and I may remark, in the first place, that all these peroxides are endowed with certain properties--obviously common to all of them, which distinguish them from the actual, higher, salt-forming oxides, especially their easy decomposition by means of simple contact agencies; their incapacity of forming salts of the common type; and their capacity of combining with other peroxides (like the faculty which hydrogen peroxide possesses of combining with barium peroxide, discovered by Schoene). Again, we remark that some groups are especially characterised by their capacity of generating peroxides. Such is, for instance, the case in the VIth group, where we find the well-known peroxides of sulphur, chromium, and uranium; so that further investigation of peroxides will probably establish a new periodic function, foreshadowing that molybdenum and wolfram will assume peroxide forms with comparative readiness. To appreciate the constitution of such peroxides, it is enough to notice that the peroxide form of sulphur (so-called persulphuric acid) stands in the same relation to sulphuric acid as hydrogen peroxide stands to water:--
H(OH), or H2O, responds to (OH)(OH), or H2O2,
and so also--
H(HSO4), or H2SO4 responds to (HSO4)(HSO4), or H2S2O8.
Similar relations are seen everywhere, and they correspond to the principle of substitutions which I long since endeavoured to represent as one of the chemical generalisations called into life by the periodic law. So also sulphuric acid, if considered with reference to hydroxyl, and represented as follows--
HO(SO2OH),
has its corresponding compound in dithionic acid--
(SO2OH)(SO2OH), or H2S2O6.
Therefore, also, phosphoric acid, HO(POH2O2), has, in the same sense, its corresponding compound in the subphosphoric acid of Saltzer:--
(POH2O2)(POH2O2), or H4P2O6;
and we must suppose that the peroxide compound corresponding to phosphoric acid, if it be discovered, will have the following structure:--
(H2PO4)2 or H2P4O8 = 2 H2O + 2 PO3.[56]
As far as is known at present, the highest form of peroxides is met with in the peroxide of uranium, UO4, prepared by Fairley;[57] while OsO4 is the highest oxide giving salts. The line of argument which is inspired by the periodic law, so far from being weakened by the discovery of peroxides, is thus actually strengthened, and we must hope that a further exploration of the region under consideration will confirm the applicability to chemistry generally of the principles deduced from the periodic law.
Permit me now to conclude my rapid sketch of the oxygen compounds by the observation that the periodic law is especially brought into evidence in the case of the oxides which constitute the immense majority of bodies at our disposal on the surface of the earth.
The oxides are evidently subject to the law, both as regards their chemical and their physical properties, especially if we take into account the cases of polymerism which are so obvious when comparing CO2 with SinO2n. In order to prove this I give the densities s and the specific volumes v of the higher oxides of two short periods. To render comparison easier, the oxides are all represented as of the form R2On. In the column headed D the differences are given between the volume of the oxygen compound and that of the parent element, divided by n, that is, by the number of atoms of oxygen in the compound:--[58]
| s. | v. | Δ. | | s. | v. | Δ.
|
|---|
| Na2O | 2.6 | 24 | -22 | K2O | 2.7 | 35 | -55
|
| Mg2O2 | 3.6 | 22 | -3 | Ca2O | 3.15 | 36 | -7
|
| Al2O3 | 4.0 | 26 | +1.3 | Sc2O3 | 3.86 | 35 | 0
|
| Si2O4 | 2.65 | 45 | 5.2 | Li2O4 | 4.2 | 38 | +5 | [sic. Ti2O4 is meant for Li2O4.--CJG]
|
| P2O5 | 2.39 | 59 | 6.2 | V2O5 | 3.49 | 52 | 6.7
|
| S2O6 | 1.96 | 82 | 8.7 | Cr2O6 | 2.74 | 73 | 9.5 |
I have nothing to add to these figures, except that like relations appear in other periods as well. The above relations were precisely those which made it possible for me to be certain that the relative density of eka-silicon oxide would be about 4.7; germanium oxide, actually obtained by Winckler, proved, in fact, to have the relative density 4.703.[59]
The foregoing account is far from being an exhaustive one of all that has already been discovered by means of the periodic law telescope in the boundless realms of chemical evolution. Still less is it an exhaustive account of all that may yet be seen, but I trust that the little which I have said will account for the philosophical interest attached in chemistry to this law. Although but a recent scientific generalisation, it has already stood the test of laboratory verification and appears as an instrument of thought which has not yet been compelled to undergo modification[60]; but it needs not only new applications, but also improvements, further development, and plenty of fresh energy. All this will surely come, seeing that such an assembly of men of science as the Chemical Society of Great Britain has expressed the desire to have the history of the periodic law described in a lecture dedicated to the glorious name of Faraday.
Notes
[1]The notion of progress as a "steady onward movement" was a common one in the 19th-century, and was applied to science as well as to social and political institutions. Mendeleev shared in this spirit of his time.
[2]The practice of science in general and chemistry in particular certainly changed in the course of the 19th century from a domain of independently wealthy amateurs to one of professionals working in communities of scholars. Mendeleev goes on to emphasize the crucial role of experimentation in the development of chemistry.
The implication that chemistry had but lately come to value empirical evidence is puzzling, however. Boyle's Sceptical Chymist [Boyle 1661] reveals an empirical and skeptical tradition more than 200 years before this speech, Lavoisier's "chemical revolution" was essentially complete 100 years before, and the wrong turn of the phlogiston theory in between was not due to disregard of experimentation.
[3]Avogadro's hypothesis that equal volumes of gas contain equal numbers of molecules [Avogadro 1811] is treated in detail earlier in chapter 9. André-Marie Ampère proposed a similar hypothesis shortly thereafter [Ampère 1814], but he suggested that molecules of elementary gases contain four atoms. Charles Gerhardt applied these ideas to the questions of chemical formulas [Gerhardt 1843], but atomic weights based on his work were still error-plagued. Avogadro's hypothesis was not widely accepted until Stanislao Cannizzaro (view portrait at Università di Roma "La Sapienza.") carefully overcame objections to it [Cannizzaro 1858]. Cannizzaro's work brought order to the confused situation regarding atomic weights in the early 1860s. It was, as Mendeleev notes below, a necessary condition for the development of classifications of the elements based on atomic weight.
[4]At the time Mendeleev spoke, a spectroscope was an instrument which analyzed the light given off by an incandescent body. The fact that different elements have different characteristic spectra made the spectroscope a sensitive tool which already allowed researchers to determine whether a particular substance was a new chemical element or a known one. [Kirchhoff & Bunsen 1860] Since that time, the practice of spectroscopy has expanded to include not only visible light but other electromagnetic radiation from radio waves to X-rays, and to analyze both emitted and absorbed radiation. It has fulfilled many of the hopes Mendeleev lists: astrochemists use spectroscopy to analyze the chemical composition of distant stars and interstellar dust, and spectra from different bands of wavelengths provide information about the nature of both atoms and the molecules into which atoms associate.
[5]Scientific communication is typically terse and prosaic, not given to flowery metaphors. A sentence such as this is worth highlighting as an exception. Similarly, Mendeleev employs carpentry metaphors below. Such exceptions are more frequently found in speech (such as this) than in written articles.
[6]Among the changes in the culture of science in the 19th century was the rise of discipline-specific scientific societies. Mendeleev's first publication on the periodic system was in the first volume of the new Russian Chemical Society's journal in 1869. Even the Chemical Society of London (now the Royal Society of Chemistry), whose journal published this address, goes back only to 1841. Interdisciplinary scientific societies such as Britain's Royal Society and the French Academy of Sciences are much older, though, dating back to the 17th century.
[7]See the previous chapter for comments on items 1-7, which correspond to the first seven points in the German abstract of Mendeleev's 1869 paper.
[8]The printed speech in Mendeleev 1889 says barium and iron, but it is obviously in error: boron (B, not Ba) and fluorine (F, not Fe) are meant. Mendeleev 1869 lists the symbols B and F for this item rather than the names of the elements.
[9]In the middle of the 19th century, there was no agreement among chemists about several concepts of fundamental importance to the science. The concepts of atom (the smallest unit of any chemical element) and molecule (the smallest unit of a chemical compound which retains the properties of that compound) were not consistently distinguished. Disagreements about chemical formulas led to disagreements about atomic weights and vice versa. Even the existence of physical atoms was doubted by many prominent chemists, which naturally led to ambiguity about what the terms "atom" or "atomic weight" could mean in a chemical context. Many of the leading chemists of Europe assembled in Karlsruhe, Germany, in 1860 to try to reach agreement on these fundamentals. (Mendeleev was present at Karlsruhe, but he was not a leading figure in the proceedings.) [Wurtz] The meeting itself did not produce agreement; however, the clear exposition of Cannizzaro [Cannizzaro 1858] distributed at the Congress in pamphlet form cleared confusion about most atomic weights within a few years.
[10]The rules Dalton proposed for chemical formulas [Dalton 1808] were arbitrary, and therefore based on a convention rather than established empirically. Other sets of atomic weights in use in the mid-19th century were based on other conventions. The introduction of the concept of "equivalent" (or equivalent weight) allowed chemists to compile ratios of combining weights without tying those weights to the concept of atoms. Thus 39 grams of potassium (K) is equivalent to 20 grams of calcium (Ca) because those masses of those elements combine with the same mass of oxygen. Equivalent weights were not the same as atomic weights, however, because equivalents often referred to different numbers of atoms; for example, 20 grams of calcium has only half as many atoms as 39 grams of potassium. The terms equivalent and atomic weight were frequently used interchangeably, however, adding to the confusion addressed at Karlsruhe.
[11]See Dumas 1857. In organic chemistry the concept of radicals was helpful in rationalizing the regular differences in weight of similar compounds. For example, any of the radicals CH3, C2H5, C3H7, etc., could replace a single H atom in an organic compound and the result would be a heavier compound with similar properties. The weights of these radicals vary regularly, for the next radical in the series differs from the previous one by a unit of fixed weight, namely CH2. (I have rendered the formulas in modern notation, which is not the notation most commonly used in the first half of the 19th century.) The analogy of families of organic radicals to families of related elements was a natural one. The weights of related elements do not vary with such regularity, though.
By the way, notice that the numbers in the Dumas table are equivalent weights, not atomic weights. (See the numbers for Ca, Sr, and Ba.) Relationships between weights of elements in the same family could still be seen when equivalents were used, for equivalents of similar elements refer to the same number of atoms. As mentioned above, however, that number of atoms is different for different families, obscuring the relationship between families.
[12]"Es ist wohl kaum anzunehmen, dass alle im Vorhergehenden hervorgehobenen Beziehungen zwischen den Atomgewichten (oder Aequivalenten) in chemischen Verhältnissen einander ähnliche Elemente bloss zufällig sind. Die Auffindung der in diesen Zahlen gesetzlichen Beziehungen müssen wir jedoch der Zukunft überlassen." [Mendeleev's note --CJG]
[13]A. E. Béguyer de Chancourtois was a minerologist and geologist at the Paris École des Mines. He proposed a natural system of classification embodied in a graphical representation he dubbed "Vis Tellurique." By plotting atomic weights along a helical curve whose base has a circumference of 16, analogous elements lie in vertical lines [de Chancourtois 1862].
I find de Chancourtois' work interesting more in the historiography of the periodic law than in its actual history. He presented his work to the French Academy of Sciences in 1862 and 1863, and extracts of his presentations were published in Comptes Rendus. The work had no effect on the subsequent development of the periodic system. Several reasons have been given for this lack of influence: de Chancourtois was a geologist, not a chemist; the Comptes Rendus articles did not contain the diagram which embodies the classification; the crucial diagram was available only in an obscure monograph [de Chancourtois 1863].
I would contend, in addition, that one can only find a periodic classification of the elements in the vis tellurique if one already knows about the periodic system. The vis tellurique included not only all the elements, but also several radicals, and some oxides, acids, alloys, and other compounds. Some substances appear in several places on the figure with different atomic weights or other characteristic numbers. De Chancourtois wrote that not only vertical lines but helices through or near several substances would also signify some sort of relations among the properties of the substances. He also referred to groups of elements which make little sense chemically (e.g., bromine, iodine, copper, and lead).
The vis tellurique was "rediscovered" around 1889. Mendeleev's mention in the Faraday lecture was followed by an article in Nature which included a translation of the first Comptes Rendus article and a much simplified figure showing only elements and their vertical relationships [Hartog 1889]. Soon afterwards, the French chemists Lecoq de Boisbaudran and de Lapparent also published a simplified version of de Chancourtois' diagram, along with a brief description of the classification [Lecoq de Boisbaudran & de Lapparent 1891]. More recently, J. W. van Spronsen counted de Chancourtois among six independent discoverers of the periodic system [van Spronsen 1969b].
[14]See chapter 11 of this volume for a detailed examination of Newlands' classifications.
[15]What Mendeleev cites here is not an octave (which is analogous to a row in a modern periodic table), but a family of elements (analogous to a column of a modern table). Newlands politely corrected Mendeleev's terminological lapse, noting that it was like calling all the A keys on a piano members of the first octave. [Newlands 1890] Moreover, I argue elsewhere [Giunta 1999] that Newlands' rows were not meant to be coextensive with families of elements. This assertion would acquit Newlands of the charge of misclassifying such obviously disparate elements, but would also observe that the law of octaves was rather indefinite about what elements it did group together.
[16]To judge from J. A. R. Newlands' work, On the Discovery of the Periodic Law, London, 1884, p. 149; "On the Law of Octaves" (from the Chemical News, 12, 83, August 18, 1865.) [Mendeleev's note --CJG]
[17]V, Nb, and Ta constitute one group (column) in the modern periodic table which lies just to the left of the group Cr, Mo, and W; similarly, P, As, and Sb lie in the same column just to the left of S, Se, and Te.
[18]Mendeleev has already called the periodic system a generalization several times. Induction is the term philosophers give to the sort of reasoning that generalizes from specific instances to wider principles, laws, or classifications. Induction is of particular importance in science, which often strives to find regularities among observed details. The periodic system is certainly the result of inductive reasoning, of generalizations from atomic weight patterns in groups of related elements and from repetition of properties in elements arranged in atomic weight order. Yet there was nothing automatic about this inductive process: the periodic system did not generalize itself.
[19]That is, Mendeleev drew certain conclusions from the periodic system which were contradictory to previously accepted notions--mainly changes in accepted atomic weights. Stephen Brush calls these sorts of predictions contrapredictions, and notes that some philosophers of science give particular weight to such risky predictions in evaluating a new scientific theory or hypothesis [Brush 1996]. Indeed, a new theory which sees phenomena that other theories either fail to see or see incorrectly appears deserving of adoption if its predictions are borne out; otherwise, it would quickly be discarded.
[20]In mathematics a periodic function is a function (a rule for associating a value with any given value) whose values repeat regularly. The most familiar periodic functions are trigonometric functions such as sine and cosine; in the equation y = cos x, the values of y repeat as the values of x continuously increase.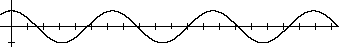 Many physical phenomena can be expressed as periodic functions of time or space. For example, the height of the surface of water in a pond after a stone was thrown into it varies periodically in both time and space: the height goes through cycles of high and low in a particular location as time passes, and at any given time, peaks and troughs alternate as one moves in space away from the point of the stone's impact. The length of a stretched spring or the magnitude of an electric field are other examples of physical properties which can be described by periodic functions of time or space. Mendeleev's use of the word harmonic later in this paragraph is similar to that of periodic; simple periodic functions such as sine and cosine are said to be harmonic in this technical sense.
Many physical phenomena can be expressed as periodic functions of time or space. For example, the height of the surface of water in a pond after a stone was thrown into it varies periodically in both time and space: the height goes through cycles of high and low in a particular location as time passes, and at any given time, peaks and troughs alternate as one moves in space away from the point of the stone's impact. The length of a stretched spring or the magnitude of an electric field are other examples of physical properties which can be described by periodic functions of time or space. Mendeleev's use of the word harmonic later in this paragraph is similar to that of periodic; simple periodic functions such as sine and cosine are said to be harmonic in this technical sense.
[21]Mendeleev states that there is a repetition of properties of the chemical elements when those elements are arranged by mass--that those properties are a periodic function of mass. He notes that no other periodic functions of mass are known in physics, and that physical phenomena which depend on mass (such as gravitational attraction) do not have recurring values as mass increases. As it turns out, the properties which seem to be periodic functions of the mass of atoms do not actually depend on their mass, but on something else to which the mass is closely related. That something else is called the atomic number [Moseley 1913, 1914], the number of units of positive electrical charge carried by the nucleus of an atom. At this time, however, there were no descriptions of the structure of an atom based on solid evidence, no notion even that atoms had a nucleus.
[22]According to their roots, both words name something which cannot be split. Individual has the same root as "indivisible": in- (not) + dividere (split, divide). Compare this to atom from a- (a-, not) + temnein (temnein, cut). Usage of atom (even in contexts outside science) tends to focus on irreducibility of atoms, while usage of individual emphasizes the special characteristics of individuals. Thus the elements can be considered individuals, while atoms refer to the smallest particles of elements.
[23]This statement is much clearer in mathematical symbols: plot y vs. x where y = sin x. An abscissa (plural abscissas or abscissae) is a distance along the x-axis of a graph; an ordinate a distance along the y-axis.
[24]In other words, periodic functions in mathematics are continuous and their graphs involve unbroken curves; however, the properties of the elements are discrete and their graphs a series of disconnected points.
[25]Mendeleev's pointed distinction between continuous and discrete functions is not appear to be very useful. There is no reason in principle why discrete points of a continuous function could not represent properties of the individual elements.
[26]Mendeleev implies that the occurence of some long periods and some short periods in the periodic system is related to the fact that the elements are discrete and not continuous. Their occurence really signifies, however, that the functions which describe properties of the elements are not strictly periodic, for they do not have a fixed period or interval of repetition. (They also are not strictly periodic because the numerical values of the properties do not strictly repeat, even at irregular intervals; they repeat only approximately.)
[27]In mathematics, the theory of numbers is concerned with relations among whole numbers, an inherently discrete subject. This seemingly obscure area includes topics such as prime numbers and factoring which actually have applicability to encryption and other practical problems in computer science (another inherently discrete subject).
[28]It would be too easy to dismiss these attempts to find a formula for the atomic weights of the elements or for some other atomic property in terms of atomic weights, because formulas such as these proved to be dead ends. Such a casual dismissal, however, would ignore that attempts to look for such regularities were inevitable, that similar attempts in other areas have sometimes been fruitful, and that sound scientific research frequently arrives at dead ends. The urge to fit a set of data to an empirical formula is part of the urge to understand the data, in the hopes that if a mathematical relationship appears to connect the data, the mathematical relationship will suggest a physical relationship. The work of Balmer [Balmer 1885] and Rydberg with frequencies of atomic spectral lines are examples of a successful search for an empirical mathematical relationship which summarized a great deal of data and proved useful to Bohr in his later formulation of a model of the atom.
[29]The masses of atoms, as already mentioned, are not the crucial variable in chemical periodicity: chemical properties depend not on the mass but on the atomic number; nor is the mass itself strictly or simply related to atomic number, even though the two are correlated. In sum, Dalton's law of multiple proportions is not related to why the masses of atoms are what they are. The valence of elements (chapter 12, note 15), however, is a key chemical property intimitely related to the structure of the atom and of the periodic table. Mendeleev is referring to valence when he talks of RX, RX2, etc. Let X represent a monovalent element, such as chlorine; then R represents consecutive elements in the periodic table, starting with any element in the leftmost column (such as potassium). So RX, RX2, etc. refer to the increasing valence of R as one moves from left to right across the table.
[30]See chapter 10 for a discussion of the search for prime matter, including Prout's hypothesis.
[31]Pythagoras lived in the 6th century BCE. He and his philosophical followers focused on number, and made fundamental advances in mathematics. The Pythagoreans sometimes carried this emphasis on number to a mystical extent, expecting nature to conform to certain preconceived numbers or ratios. Mendeleev seems to use Pythagorean as a shorthand for a philosophical attitude based on a priori expectations rather than scientific empiricism.
[32]Jean Servais Stas carried out an extensive set of chemical analyses in the hopes of vindicating Prout's "multiples" hypothesis (chapter 10); however, he observed, and in 1860 reported, just the opposite. [Stas 1860]
[33]That is, a body having a wave-length equal to 0.0005875 millimetre. [Mendeleev's note --CJG]
[34]Mendeleev is incorrect, of course, in calling helium imaginary. In 1868, J. Norman Lockyer and Edward Frankland had identified a spectral line in the sun which did not correspond to any known substance; they named the supposed source of that line (and of others subsequently identified) helium for its origin in the sun. Helium was not detected on earth until 1895, after the discovery of the chemically similar gas argon. (See the next two chapters, 14 and 15.) Before helium was found on earth and characterized, some speculated that it was the primary matter from which other elements were formed. There was no evidence for this idea, and no way to test it at that time. Yet it was not an unreasonable speculation: the idea was that the hydrogen found so abundantly in the sun and stars (also by spectral analysis) might somehow be broken down by the extreme heat of these bodies into a more fundamental form of matter.
Mendeleev's attitude toward helium and the speculations surrounding it is at least justifiable skepticism if not disbelief. That attitude would change toward the end of his life [van Spronsen 1969b], when he indulged in similar speculation about stellar elements lighter than hydrogen.
[35]He maintains (on p. 309) that the periodic law requires two new analogous elements, having atomic weights of 48 and 64, occupying positions between sulphur and selenium, although nothing of the kind results from any of the different readings of the law. [Mendeleev's note --CJG]
[36]I.e., Mendeleev maintains that the periodic classification of the elements stands on its own, and neither supports nor argues against the idea that the elements are built up (compounded) of more fundamental particles. I find this assertion unobjectionable in principle, even though we now do rationalize the periodicity of the elements as a consequence of the internal structure of atoms.
[37]The law of Avogadro is the hypothesis that equal volumes of gas contain equal numbers of molecules (chapter 9) [Avogadro 1811]. The law of specific heats is better known to modern readers as the law of Dulong and Petit [Petit & Dulong 1819], the observation that atomic heat capacities are approximately equal for all elements. Both Avogadro's hypothesis and the law of Dulong and Petit were seen as evidence supporting the atomic hypothesis (that is, the existence of discrete ultimate particles of elements), and both were important in Cannizzaro's Sunto [Cannizzaro 1858]
[38]The question of the nature of the elements, whether they are truly irreducible individuals or whether they are compounded from a more fundamental primary matter, was essentially a philosophical question rather than a scientific one at this time, for there was no good way of testing the question empirically. Mendeleev seems to discredit the idea of a primary matter by tracing it to the ancients, who had no scientific evidence for it, and linking it to notions of unity and universality, which are clearly extrascientific. Yet his arguments for the ultimate individuality of the elements are based on similarly metaphysical arguments: the distinction between discreteness and continuity simply is not relevant to the issue.
[39]It is noteworthy that the year in which Lavoisier was born (1743)--the author of the idea of elements and of the indestructibility of matter--is later by exactly one century than the year in which the author of the theory of gravitation and mass was born (1643 N.S.). The affiliation of the ideas of Lavoisier and those of Newton is beyond doubt. [Mendeleev's note --CJG]
[40]See note 11 above. The series of radicals represented in the table increase in valence from left to right, just as a series of elements in a row of the periodic table. Such an analogy between radicals and elements led some chemists to believe that elements are compounded from simpler materials, just as radicals are compounded from elements. Mendeleev warns that the analogy is not perfect, for the series of radicals decreases in mass as it increases in valence; elements, converseley, increase in mass as they increase in valence. Since Mendeleev believed that periodic properties (including valence) depended directly on mass, this observation was enough to make him wary of pushing the analogy of radicals and elements too far.
[41]See chapters 2 and 3. In fact, the concept of element did not change very much between the time of Robert Boyle (an older contemporary of Newton), and Lavoisier. The list of substances considered to be elements, however, changed substantially during that time--indeed, Boyle was reluctant even to offer an opinion on which substances might be elementary.
[42]This sentence expresses the importance of both theory (with its mental tools of logic and mathematics) and experiment (with its physical instruments) to the development of science.
[43]I foresee some more new elements, but not with the same certitude as before. I shall give one example, and yet I do not see it quite distinctly. In the series which contains Hg = 204, Pb = 206, and Bi = 208,we can guess the existence (at the place VI-11) of an element analogous to tellurium, which we can describe as dvi-tellurium, Dt having an atomic weight of 212, and the property of forming the oxide DtO3. If this element really exists, it ought in the free state to be an easily fusible, crystalline, non-volatile metal of a grey colour, having a density of about 9.3, capable of giving a dioxide, DtO2, equally endowed with feeble acid and basic properties. This dioxide must give on active oxidation an unstable higher oxide, DtO3, which should resemble in its properties PbO2 and Bi2O5. Dvi-tellurium hydride, if it be found to exist, will be a less stable compound than even H2Te. The compounds of dvi-tellurium will be easily reduced, and it will form characteristic definite alloys with other metals. [Mendeleev's note --CJG]
[44]Mendeleev's detailed predictions of these three elements can be found in Mendeleev 1871. Explicit but less detailed predictions of gallium and germanium, as well as a hint of a prediction of scandium can be seen in Mendeleev 1869. (See previous chapter.) For accounts of the discovery of these elements, see Lecoq de Boibaudran 1877, Nilson 1879, Weeks & Leicester 1968, and Winkler 1886.
The discovery of these elements had a great effect on the acceptance of the periodic system by Mendeleev's contemporaries. Indeed, the periodic law is the paramount example in chemistry of successful predictions playing an important role in the acceptance of a hypothesis [Brush 1996], even if the periodic law's successful accommodation of a great deal of chemical information played an even greater role [Scerri & Worrall 2001]. Mendeleev saw the potential that the discovery of gallium had in persuading chemists to accept his system: "If subsequent research confirms the identity of properties which I describe for eka-aluminum with those of gallium, this will be an instructive example of the utility of the periodic law." [Mendeleev 1875] And so it was, despite the initial lukewarm reaction of gallium's discoverer to Mendeleev's predictions [de Boisbaudran 1875].
The element Mendeleev predicts in his footnote to this section was discovered within 10 years [Curie & Curie 1898]. Polonium is a metal of atomic weight 210 and density 9.4 g cm-3.
[45]See the preceding chapter, note 11. Mendeleev did not know where to put these elements in his 1869 system, but quickly found places for them (usually the correct place).
[46]Let me mention another proof of the bivalency of beryllium which may have passed unnoticed, as it was published in the Russian chemical literature. Having remarked (in 1884) that the density of such solutions of chlorides of metals, MCln, as contain 200 mols. of water (or a large and constant amount of water) regularly increases as the molecular weight of the dissolved salt increases, I proposed to one of our young chemists, J. Burdakoff, that he should investigate the beryllium chloride. If its molecule is BeCl2 its weight must be = 80; and in such a case it must be heavier than the molecule of KCl = 74.5, and lighter than that of MgCl [sic: MgCl2 is intended --CJG] = 93. On the contrary, if beryllium chloride is a trichloride, BCl3 = 120, its molecule must be heavier than that of CaCl2 = 111, and lighter than that of MnCl2 = 126. Experiment has shown the correctness of the former formula, the solution BeCl2 + 200 H2O having (at 15°/4°) a density of 1.0138, this being a higher density than that of the solution KCl + 200 H2O (=1.0121), and lower than that of MgCl2 + 200 H2O (=1.0203). The bivalency of beryllium was thus confirmed in the case both of the dissolved and the vaporised chloride. [Mendeleev's note --CJG]
[47]Neither the atomic weight nor the characteristic valence of beryllium was firmly established when the periodic law was introduced. Analysis of beryllium oxide showed that the compound contained nearly twice as much oxygen as beryllium by weight. Since the atomic weight of oxygen was known to be 16, the atomic weight of Be would be about 9 if the formula of the oxide was BeO (like magnesia, MgO); however, if the formula was Be2O3 (like alumina, Al2O3), the atomic weight of Be would be about 14. Mendeleev opted for an atomic weight near 9 [Mendeleev 1869], for that would place Be in a group of elements whose oxides were analogous to magnesia (MgO). The alternative would have placed Be between carbon and nitrogen, where there was no room for it and among elements which did not resemble it.
Thus, the table provided additional information which helped fix an atomic weight. At the same time, it made a testable prediction which has since been confirmed: namely, that the chemical behavior of Be is similar to that of Mg and other members of that group. Here Mendeleev points to the atomic weight of Be as an example of the utility of the periodic law and of a successful prediction based on the law. To say that his prediction challenged a "well established" result, though, is a bit of an exaggeration. For example, the atomic weight of 9 appeared in the work of such classifiers as de Chancourtois [de Chancourtois 1863], Odling [Odling 1864], and Newlands [Newlands 1864] before Mendeleev's first periodic system. The explicitness of Mendeleev's prediction [Mendeleev 1871], however, was a contrast to the ambiguousness of, say, Newlands', which was made explicit only in 1878 [Newlands 1878].
[48]Uranium remains the heaviest element, at least among those which occur naturally on earth. As of 2002, twenty-two elements heavier than uranium have been artificially produced (elements 93-112, 114, and 116)--mainly in small quantities. Uranium also remains an element endowed with a special interest even greater than that to which Mendeleev alludes because of its radioactivity (chapters 17 and 18) and the facility with which it participates in energy-releasing nuclear reactions.
[49]This redetermination [Brauner 1883] was erroneous, as was Mendeleev's prediction. (Brauner later determined the atomic weight of tellurium yet again, obtaining and reporting a range of figures; he concluded that tellurium was not a single element. [Brauner 1889]) Given Mendeleev's belief that the order of the periodic system depended directly on atomic mass, it is hardly surprising that he sought to correct what appeared to be an instance of masses out of order. According to chemical properties, tellurium had to precede iodine, but the best measurements of atomic weight gave 128 for tellurium and 127 for iodine. Those atomic weights were correct (current values 127.6 for tellurium and 126.9 for iodine), so the atomic weight of tellurium is an example of an erroneous contraprediction. Mendeleev's classification, however, was correct, for tellurium precedes iodine in atomic number. The erroneous contraprediction was a result of an incomplete understanding of the physical bases of the periodic system.
[50]I pointed them out in the Liebig's Annalen, Supplement Band viii, 1871, p. 211. [Mendeleev's note --CJG]
[51]I.e., that the order according to chemical properties ought to be Os, Ir, Pt, Au. That is in fact the order in the current periodic table as given by atomic number (76, 77, 78, 79 respectively), as well as the order by the best available atomic weights (190.2, 192.2, 195.1, 197.0).
[52]Thus, in the typical small period of
Li, Be, B, C, N, O, F,
we see at once the progression from the alkaline metals to the acid non-metals, such as are the halogens. [Mendeleev's note --CJG]
[53]Liebig's Annalen, Erz. Bd. vii, 1870. [Mendeleev's note --CJG]
[54]A distinct periodicity can also be discovered in the spectra of the elements. Thus the researches of Hartley, Ciamician, and others have disclosed, first, the homology of the spectra of analogous elements; secondly, that the alkaline metals have simpler spectra than the metals of the following groups; and thirdly, that there is a certain likeness between the complicated spectra of manganese and iron on the one hand, and the no less complicated spectra of chlorine and bromine on the other hand, and their likeness corresponds to the degree of analogy between those elements which is indicated by the periodic law. [Mendeleev's note --CJG]
[55]Formerly it was supposed that, being a bivalent element, oxygen can enter into any grouping of the atoms, and there was no limit foreseen as to extent to which it could further enter into combination. We could not explain why bivalent sulphur, which forms compounds such as
could not also form oxides such as--
while other elements, as for instance, chlorine, form compounds such as--
Cl-O-O-O-O-K. [Mendeleev's note --CJG]
[56]In this sense, oxalic acid, (COOH)2, also corresponds to carbonic acid, OH(COOH), in the same way that dithionic acid corresponds to sulphuric acid, and subphosphoric acid to phosphoric; therefore, if a peroxide, corresponding to carbonic acid, be obtained, it will have the structure of (HCO3)2, or H2C2O6 = H2O + C2O5. So also lead must have a real peroxide, Pb2O5. [Mendeleev's note --CJG]
[57]The compounds of uranium prepared by Fairley seem to me especially instructive in understanding the peroxides. By the action of hydrogen peroxide on uranium oxide, UO3, a peroxide of uranium, UO44H2O, is obtained (U = 240) if the solution be acid; but if hydrogen peroxide act on uranium oxide in the presence of caustic soda, a crystalline deposit is obtained, which has the composition Na4UO84H2O, and evidently is a combination of sodium peroxide, Na2O2, with uranium peroxide UO4. It is possible that the former peroxide, UO44H2O, contains the elements of hydrogen peroxide and uranium peroxide, U2O7, or even U(OH)6H2O2, like the peroxide of tin recently discovered by Spring, which has the constitution Sn2O5H2O2. [Mendeleev's note --CJG]
[58]D thus represents the average increase of volume for each atom of oxygen contained in the higher salt-forming oxide. The acid oxides give, as a rule, a higher value of D, while in the case of the strongly alkaline oxides its value is usually negative. [Mendeleev's note --CJG]
[59]That is, Mendeleev was able to set up a series of empirical quantitative relationships which permitted him to interpolate predicted values of missing elements from those of elements in the same row. The fact that the periodic system was amenable to such quantitative manipulation allowed it to generate the specific and explicit predictions and explanations which are so much more convincing than vague explanations based on qualitative chemical similarity.
[60]The fact that modifications of the periodic system could be made relatively easily is an indication of the durability and utility of the periodic law. Such a modification was necessary when the family of gases containing argon and helium was discovered later in the 19th century. (See chapters 14 and 15.) The periodic system accommodated the new discoveries simply by having an additional column tacked on.
References
- André-Marie Ampère, Annales de chimie et de physique 90, 45- (1814); translated in Philosophical Magazine 45, 41-43, 109-116, 188-193, 344-349 (1815)
- Amedeo Avogadro, "Essay on a Manner of Determining the Relative Masses of the Elementary Molecules of Bodies, and the Proportions in Which They Enter into These Compounds," Journal de Physique 73, 58-78 (1811), translated in Alembic Club Reprint No. 4, (Edinburgh, 1890); annotated excerpts.
- Johann Jacob Balmer, "Note on the Spectral Lines of Hydrogen," Annalen der Physik und Chemie 25, 80-85 (1885)
- Paul-Èmile Lecoq de Boisbaudran, "Sur quelques propriétés de gallium," Comptes Rendus 81, 1100-1105 (1875)
- Paul-Èmile Lecoq de Boisbaudran & Albert de Lapparent, "Sur une réclamation de priorité en faveur de M. de Chancourtois, relativement aux relations numériques des poids atomiques," Comptes Rendus 112, 77-81 (1891)
- Robert Boyle, The Sceptical Chymist, London, 1661; annotated excerpts
- Bohuslav Brauner, "Ueber das Atomgewicht des Tellurs," Berichte 16, 3055-3056 (1883)
- Bohuslav Brauner, "Experimental Researches on the Periodic Law. Part I. Tellurium," Journal of the Chemical Society (London) 56, 382-411 (1889)
- Stephen G. Brush, "The Reception of Mendeleev's Periodic Law in America and Britain," Isis 87 595-628 (1996)
- Stanislao Cannizzaro, Nuovo Cimento 7, 321-366 (1858); translated as Alembic Club Reprints #18, Sketch of a Course of Chemical Philosophy (Edinburgh: Alembic Club, 1910)
- A. E. Béguyer de Chancourtois, "Mémoire sur un classement naturel des corps simples ou radicaux appelé vis tellurique," Comptes Rendus 54, 757-761 (1862)
- A. E. Béguyer de Chancourtois, Vis Tellurique: Classement Naturel des corps simples ou radicaux (Paris: Mallet-Bachelier, 1863)
- Pierre Curie & Marie Curie, "On a New Radioactive Substance Contained in Pitchblende," Comptes Rendus 127, 175-178 (1898)
- John Dalton, A New System of Chemical Philosophy Part I (Manchester, 1808), p. 214; annotated excerpts.
- Jean Baptiste André Dumas, "Mémoire sur les équivalents des corps simples," Comptes Rendus 45, 709-731 (1857)
- Charles Gerhardt, "Considerations sur les équivalents de quelques corps simples et composé," Ann. Chim. Phys. 7, 129-143; 8, 239-245 (1843)
- Carmen J. Giunta, "J. A. R. Newlands' Classification of the Elements: Periodicity, but no System," Bulletin for the History of Chemistry 24, 24-31 (1999) A Philosophical Commentary on this article was published in this journal by Eric R. Scerri [26, 124-129 (2001)] along with the author's response [26, 130-132 (2001)].
- P. J. Hartog, "A First Foreshadowing of the Periodic Law," Nature 46, 186-188 (1889)
- Gustav Kirchhoff & Robert Bunsen, "Chemical Analysis by Observation of Spectra," Annalen der Physik und der Chemie (Poggendorff) 110, 161-189 (1860)
- Paul Émile Lecoq de Boisbaudran, "About a New Metal, Gallium," Annales de Chimie 10, 100-141 (1877)
- Dmitrii Mendeleev, "On the Relationship of the Properties of the Elements to their Atomic Weights," Zhurnal Russkoe Fiziko-Khimicheskoe Obshchestvo 1, 60-77 (1869); abstracted in Zeitschrift für Chemie 12, 405-406 (1869); annotated abstract
- Dmitrii Mendeleev, Zhurnal Russkoe Fiziko-Khimicheskoe Obshchestvo 3, 25 (1871); German version, "Die periodische Gesetzmässigkeit der chemischen Elemente," Annalen der Chemie und Pharmacie Supplement 8, 133-229 (1872); table from this paper
- Dmitrii Mendeleev, "Remarques à propos de la découverte du gallium," Comptes Rendus 81, 969-972 (1875)
- Dmitrii Mendeleev, "The Periodic Law of the Chemical Elements," Journal of the Chemical Society (London) 55, 634-656 (1889); annotated above
- Henry G. J. Moseley, "The High Frequency Spectra of the Elements," Philosophical Magazine 26, 1024-1034 (1913) and 27, 703-713 (1914)
- J. A. R. Newlands, "Relations between equivalents," Chemical News 10, 59-60 (1864); annotated text
- J. A. R. Newlands, "On the Law of Octaves," Chemical News, 12, 83 (1865); annotated text
- J. A. R. Newlands, "On the Periodic Law," Chemical News 38, 106-107 (1878)
- J. A. R. Newlands, "On the Periodic Law," Chemical News 61, 136 (1890)
- Lars Fredrick Nilson, "About Ytterbine, the New Earth of Marignac," Comptes Rendus 88, 642-664 (1879)
- William Odling, "On the Proportional Numbers of the Elements," Quarterly Journal of Science 1, 642-648 (1864)
- Alexis-Thérèse Petit & Pierre-Louis Dulong, "Recherches sur quelques points importants de la Théorie de la Chaleur," Annales de Chimie et de Physique 10, 395-413 (1819); contemporary translation from Annals of Philosophy 14, 189-198 (1819)
- Eric R. Scerri & John Worrall, "Prediction and the Periodic Table," Studies in History and Philosophy of Science 32, 407-452 (2001)
- Jean Servais Stas, "Researches on the Mutual Relations of Atomic Weights," Bulletin de l'Académie Royale de Belgique 10, 208-336 (1860); translated and excerpted in Alembic Club Reprints #20, Prout's Hypothesis , (Edinburgh, 1932)
- J. W. van Spronsen, Journal of Chemical Education 46, 136-139 (1969a)
- J. W. van Spronsen, The Periodic System of Chemical Elements: a History of the First Hundred Years (Amsterdam: Elsevier, 1969b)
- Mary Elvira Weeks & Henry M. Leicester, Discovery of the Elements, 7th ed. (Easton, PA: Journal of Chemical Education, 1968)
- Clemens Winkler, "Germanium, Ge, a New Nonmetallic Element," Berichte der Deutschen Chemischen Gesellschaft 19, 210-211 (1886)
- Charles-Adolphe Wurtz, "Account of the Sessions of the International Congress of Chemists in Karlsruhe, on 3, 4, and 5 September 1860," originally in Richard Anschütz, August Kekulé, 2 vols. (Berlin: Verlag Chemie, 1929) as Appendix VIII (pp. 671-88 of vol. 1); English translation by John Greenberg and William Clark published in Mary Jo Nye, The Question of the Atom (Los Angeles: Tomash, 1984)
 Back to the top of the table of contents of Elements and Atoms.
Back to the top of the table of contents of Elements and Atoms.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.
 Many physical phenomena can be expressed as periodic functions of time or space. For example, the height of the surface of water in a pond after a stone was thrown into it varies periodically in both time and space: the height goes through cycles of high and low in a particular location as time passes, and at any given time, peaks and troughs alternate as one moves in space away from the point of the stone's impact. The length of a stretched spring or the magnitude of an electric field are other examples of physical properties which can be described by periodic functions of time or space. Mendeleev's use of the word harmonic later in this paragraph is similar to that of periodic; simple periodic functions such as sine and cosine are said to be harmonic in this technical sense.
Many physical phenomena can be expressed as periodic functions of time or space. For example, the height of the surface of water in a pond after a stone was thrown into it varies periodically in both time and space: the height goes through cycles of high and low in a particular location as time passes, and at any given time, peaks and troughs alternate as one moves in space away from the point of the stone's impact. The length of a stretched spring or the magnitude of an electric field are other examples of physical properties which can be described by periodic functions of time or space. Mendeleev's use of the word harmonic later in this paragraph is similar to that of periodic; simple periodic functions such as sine and cosine are said to be harmonic in this technical sense.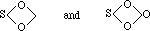
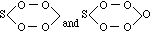
 Back to the top of the table of contents of Elements and Atoms.
Back to the top of the table of contents of Elements and Atoms.