Henry Cavendish (1731-1810)
Experiments on Air
Philosophical Transactions 75, 372 (1785)
In a paper, printed in the last volume of the Philosophical Transactions, in which I gave my reasons for thinking that the diminution produced in atmospheric air by phlogistication, is not owing to the generation of fixed air, I said it seemed most likely, that the phlogistication of air by the electric spark was owing to the burning of some inflammable matter in the apparatus; and that the fixed air, supposed to be produced in that process, was only separated from that inflammable matter by the burning. At that time, having made no experiments on the subject myself, I was obliged to form my opinion from those already published; but I now find, that though I was right in supposing the phlogistication of the air does not proceed from phlogiston communicated to it by the electric spark, and that no part of the air is converted into fixed air; yet that the real cause of the diminution is very different from what I suspected, and depends on the conversion of phlogisticated air into nitrous acid.
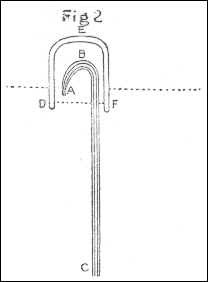
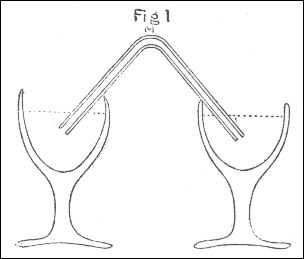 The apparatus used in making the experiments was as follows: The air through which the spark was intended to be passed, was confined in a glass tube M, bent to an angle, as in fig. 4, pl. 1, which, after being filled with quicksilver, was inverted into two glasses of the same fluid, as in the figure. The air to be tried was then introduced by means of a small tube, such as is used for thermometers, bent in the manner represented by ABC, fig. 5, the bent end of which, after being previously filled with quicksilver, was introduced, as in the figure, under the glass DEF, inverted into water, and filled with the proper kind of air, the end C of the tube being kept stopped by the finger: then, on removing the finger from C, the quicksilver in the tube descended in the leg BC, and its place was supplied with air from the glass DEF. Having thus got the proper quantity of air into the tube ABC, it was held with the end C uppermost, and stopped with the finger; and the end A, made smaller for that purpose, being introduced into one end of the bent tube M, fig. 4, the air, on removing the finger from C, was forced into that tube by the pressure of the quicksilver in the leg BC. By these means I was enabled to introduce the exact quantity I pleased of any quantity of soap-lees, or any other liquor which I wanted to be in contact with the air.
The apparatus used in making the experiments was as follows: The air through which the spark was intended to be passed, was confined in a glass tube M, bent to an angle, as in fig. 4, pl. 1, which, after being filled with quicksilver, was inverted into two glasses of the same fluid, as in the figure. The air to be tried was then introduced by means of a small tube, such as is used for thermometers, bent in the manner represented by ABC, fig. 5, the bent end of which, after being previously filled with quicksilver, was introduced, as in the figure, under the glass DEF, inverted into water, and filled with the proper kind of air, the end C of the tube being kept stopped by the finger: then, on removing the finger from C, the quicksilver in the tube descended in the leg BC, and its place was supplied with air from the glass DEF. Having thus got the proper quantity of air into the tube ABC, it was held with the end C uppermost, and stopped with the finger; and the end A, made smaller for that purpose, being introduced into one end of the bent tube M, fig. 4, the air, on removing the finger from C, was forced into that tube by the pressure of the quicksilver in the leg BC. By these means I was enabled to introduce the exact quantity I pleased of any quantity of soap-lees, or any other liquor which I wanted to be in contact with the air.
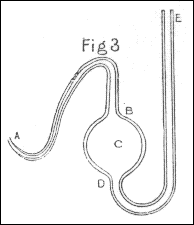
In one case however, in which I wanted to introduce air into the tube many times in the same experiment, I used the apparatus represented in fig. 6, consisting of a tube AB of a small bore, a ball C, and a tube DE of a larger bore. This apparatus was first filled with quicksilver; and then the ball C and the tube AB were filled with air, by introducing the end A under a glass inverted into water, which contained the proper kind of air, and drawing out the quicksilver from the leg ED by a syphon. After being thus furnished with air, the apparatus was weighed, and the end A introduced into one end of the tube M, and kept there during the experiment; the way of forcing air out of this apparatus into the tube being by thrusting down the tube ED a wooden cylinder of such a size as almost to fill up the whole bore, and by occasionally pouring quicksilver into the same tube, to supply the place of that pushed into the ball C. After the experiment was finished, the apparatus was weighed again, which showed exactly how much air had been forced into the tube M during the whole experiment; it being equal in bulk to a quantity of quicksilver, whose weight was equal to the increase of weight of the apparatus.
The bore of the tube M used in most of the following experiments, was about 1/10 of an inch; and the length of the column of air, occupying the upper part of the tube, was in general from 1-1/2 to 3/4 of an inch. It is scarcely necessary to inform any one used to electrical experiments, that in order to force an electrical spark through the tube, it was necessary, not to make a communication between the tube and the conductor, but to place an insulated ball at such a distance from the conductor as to receive a spark from it, and to make a communication between the ball and the quicksilver in one of the glasses, while the quicksilver in one of the glasses, while the quicksilver in the other glass communicated with the ground. I now proceed to the experiments.
When the electric spark was made to pass through common air, included between short columns of a solution of litmus, the solution acquired a red colour, and the air was diminished, conformably to what was observed by Dr. Priestley. When lime-water was used, instead of the solution of litmus, and the spark was continued till the air could be no further diminished, not the least cloud could be perceived in the lime-water; but the air was reduced to 2/3 of its original bulk; which is a greater diminution than it could have suffered by mere phlogistication, as that is very little more than 1/6 of the whole. The experiment was next repeated with some impure dephlogisticated air. The air was very much diminished, but without the least cloud being produced in the lime-water. Neither was any cloud produced when fixed air was let up to it; but on the further addition of a little caustic volatile alkali, a brown sediment was immediately perceived.
Hence we may conclude, that the lime-water was saturated by some acid formed during the operation; as in the case it is evident that no earth could be precipitated by the fixed air alone, but that caustic volatile alkali, on being added, would absorb the fixed air, and thus becoming mild, would immediately precipitate the earth; whereas, if the earth in the lime-water had not been saturated with an acid, it would have been precipitated by the fixed air. As to the brown colour of the sediment, it most likely proceeded from some of the quicksilver having been dissolved. It must be observed, that if any fixed air, as well as acid, had been generated in these two experiments with the lime-water, a cloud must have been at first perceived in it, though that cloud would afterwards disappear by the earth being re-dissolved by the acid; for till the acid produced was sufficient to dissolve the whole of the earth, some of the remainder would be precipitated by the fixed air; so that we may safely conclude, that no fixed air was generated in the operation.
When the air is confined by soap-lees, the diminution proceeds rather faster than when it is confined by lime-water; for which reason, as well as on account of their containing so much more alkaline matter in proportion to their bulk, soap-lees seemed better adapted for experiments designed to investigate the nature of this acid, than lime-water. I accordingly made some experiments to determine what degree of purity the air should be of, in order to be diminished most readily, and to the greatest degree; and I found that when good dephlogisticated air was used, the diminution was but small; when perfectly phlogisticated air was used, no sensible diminution took place; but when 5 parts of pure dephlogisticated air were mixed with 3 parts of common air, almost the whole of the air was made to disappear. It must be considered, that common air consists of 1 part of dephlogisticated air, mixed with 4 of phlogisticated; so that a mixture of 5 parts of pure dephlogisticated air, and 3 of common air, is the same thing as a mixture of 7 parts of dephlogisticated air with 3 of phlogisticated.
Having made these previous trials, I introduced into the tube a little soap-lees, and then let up some dephlogisticated and common air, mixed in the above-mentioned proportion, which rising to the top of the tube M, divided the soap-lees into its two legs. As fast as the air was diminished by the electric spark, I continued adding more of the same kind, till no further diminution took place: after which a little pure dephlogisticated air, and after that a little common air, were added, in order to see whether the cessation of diminution was not owing to some imperfection in the proportion of the two kinds of air to each other; but without effect.[1] The soap-lees being then poured out of the tube, and separated from the quicksilver, seemed to be perfectly neutralized, as they did not at all discolour paper tinged with the juice of blue flowers. Being evaporated to dryness, they left a small quantity of salt, which was evidently nitre, as appeared by the manner in which paper, impregnated with a solution of it, burned.
For more satisfaction, I tried this experiment over again on a larger scale. About 5 times the former quantity of soap-lees were now let up into a tube of a larger bore; and a mixture of dephlogisticated and common air, in the same proportions as before, being introduced by the apparatus represented in fig. 6, the spark was continued till no more air could be made to disappear. The liquor, when poured out of the tube, smelled evidently of phlogisticated nitrous acid, and being evaporated to dryness, yielded 1-4/10 gr. of salt, which is pretty exactly equal in weight to the nitre which that quantity of soap-lees would have afforded if saturated with nitrous acid. This salt was found, by the manner in which paper dipped into a solution of it burned, to be true nitre. It appeared, by the test of terra ponderosa salita, to contain not more vitriolic acid than the soap-lees themselves contained, which was excessively little; and there is no reason to think that any other acid entered into it, except the nitrous. A circumstance however occurred, which at first seemed to show that this salt contained some marine acid; namely, an evident precipitation took place when a solution of silver was added to some of it dissolved in water; though the soap-lees used in its formation were perfectly free from marine acid, and though, to prevent all danger of any precipitate being formed by an excess of alkali in it, some purified nitrous acid had been added to it, previous to the addition of the solution of silver. On consideration however I suspected that this precipitation might arise from the nitrous acid in it being phlogisticated; and therefore I tried whether nitre, much phlogisticated, would precipitate silver from its solution. For this purpose I exposed some nitre to the fire, in an earthen retort, till it had yielded a good deal of dephlogisticated air; and then, having dissolved it in water, and added to it some well purified spirit of nitre till it was sensibly acid, in order to be certain that the alkali did not predominate, I dropped into it some solution of silver, which immediately made a very copious precipitate. This solution however being deprived of some of its phlogiston by evaporation to dryness, and exposure for a few weeks to the air, lost the property of precipitating silver from its solution; a proof that this property depended only on its phlogistication, and not on its having absorbed sea-salt from the retort, or by any other means. Hence it is certain that nitre, when much phlogisticated, is capable of making a precipitate with a solution of silver; and therefore there is no reason to think that the precipitate, which our salt occasioned with a solution of silver, proceeded from any other cause than that of its being phlogisticated; especially as it appeared by the smell, both on first taking it out of the tube, and on the addition of the spirit of nitre, previous to dropping in the solution of silver, that the acid in it was much phlogisticated. This property of phlogisticated nitre is worth the attention of chemists; as otherwise they may sometimes be led into mistakes, in investigating the presence if marine acid by a solution of silver.
In the above-mentioned paper I said, that when nitre is detonated with charcoal, the acid is converted into phlogisticated air; that is, into a substance which, as far as I could perceive, possesses all the properties of the phlogisticated air of our atmosphere; from which I concluded, that phlogisticated air is nothing else than nitrous acid united to phlogiston. According to this conclusion, phlogisticated air ought to be reduced to nitrous acid by being deprived of its phlogiston. But as dephlogisticated air is only water deprived of phlogiston, it is plain, that adding dephlogisticated air to a body, is equivalent to depriving it of phlogiston, and adding water to it; and therefore phlogisticated air ought also to be reduced to nitrous acid, by being made to unite to, or form a chemical combination with dephlogisticated air; only the acid formed this way will be more dilute, than if the phlogisticated air was simply deprived of phlogiston.
This being premised, we may safely conclude, that in the present experiments the phlogisticated air was enabled, by means of the electrical spark, to unite to, or form a chemical combination with the dephlogisticated air, and was thus reduced to nitrous acid, which united to the soap-lees, and formed a solution of nitre; for in these experiments those two airs actually disappeared, and nitrous acid was actually formed in their stead; and as moreover it has also been just shown, from other circumstances, that phlogisticated air must form nitrous acid, when combined with dephlogisticated air, the above-mentioned opinion seems to be sufficiently established. A further confirmation of it is that, as far as I can perceive, no diminution of air is produced when the electric spark is passed either through pure dephlogisticated air, or through perfectly phlogisticated air; which indicates the necessity of a combination of these two airs to produce the acid. It was also found in the last experiment, that the quantity of nitre procured was the same that the soap-lees would have produced if saturated with nitrous acid; which shows that the production of the nitre was not owing to any decomposition of the soap-lees. It may be worth remarking, that whereas in the detonation of nitre with inflammable substances, the acid unites to phlogiston, and forms phlogisticated air, in these experiments the reverse of this process was carried on; namely, the phlogisticated air united to the dephlogisticated, which is equivalent to being deprived of its phlogiston, and was reduced to nitrous acid.
In the above-mentioned paper I also gave my reasons for thinking that the small quantity of nitrous acid, produced by the explosion of dephlogisticated and inflammable air, proceeded from a portion of phlogisticated air mixed with the dephlogisticated, which I supposed was deprived of its phlogiston, and turned into nitrous acid, by the action of the dephlogisticated air on it, assisted by the heat of the explosion. This opinion, as must appear to every one, is confirmed in a remarkable manner by the foregoing experiments; as from them it is evident that dephlogisticated air is able to deprive phlogisticated air of its phlogiston, and reduce it into acid, when assisted by the electric spark; and therefore it is not extraordinary that it should do so when assisted by the heat of the explosion.
The soap-lees used in the foregoing experiments were made from salt of tartar, prepared without nitre; and were of such a strength as to yield 1/10 of their weight of nitre when saturated with nitrous acid. The dephlogisticated air also was prepared without nitre, that used in the first experiment with the soap-lees being procured from the black powder formed by the agitation of quicksilver mixed with lead,[2] and that used in the latter from turbith mineral. In the first experiment, the quantity of soap-lees used was 35 measures, each of which was equal in bulk to 1 grain of quicksilver; and that of the air absorbed was 416 such measures of phlogisticated air, and 914 of dephlogisticated. In the 2d experiment, 178 measures of soap-lees were used, and they absorbed 1920 of phlogisticated air, and 4860 of dephlogisticated. It must be observed however, that in both experiments some air remained in the tube uncondensed, whose degree of purity I had no way of trying; so that the proportion of each species of air absorbed is not known with much exactness.
As far as the experiments hitherto published extend, we scarcely know more of the nature of the phlogisticated part of our atmosphere, than that it is not diminished by lime-water, caustic alkalis, or nitrous air; that it is unfit to support fire, or maintain life in animals; and that its specific gravity is not much less than that of common air; so that, though the nitrous acid, by being united to phlogiston, is converted into air possessed of these properties, and consequently, though it was reasonable to suppose that part at least of the phlogisticated air of the atmosphere consists of this acid united to phlogiston, yet it might fairly be doubted whether the whole is of this kind, or whether there are not in reality many different substances confounded together by us under the name of phlogisticated air. I therefore made an experiment to determine, whether the whole of a given portion of the phlogisticated air of the atmosphere could be reduced to nitrous acid, or whether there was not a part of a different nature from the rest, which would refuse to undergo that change. The foregoing experiments indeed in some measure decided this point, as much the greatest part of the air let up into the tube lost its elasticity; yet, as some remained unabsorbed, it did not appear for certain whether that was of the same nature as the rest or not. For this purpose I diminished a similar mixture of dephlogisticated and common air, in the same manner as before, till it was reduced to a small part of its original bulk. I then, in order to decompound as much as I could of the phlogisticated air which remained in the tube, added some dephlogisticated air to it, and continued the spark till no further diminution took place. Having by these means condensed as much as I could of the phlogisticated air, I let up some solution of liver of sulphur to absorb the dephlogisticated air; after which only a small bubble of air remained unabsorbed, which certainly was not more than 1/120 of the bulk of the phlogisticated air let up into the tube; so that if there is any part of the phlogisticated air of our atmosphere which differs from the rest, and cannot be reduced to nitrous acid, we may safely conclude, that it is not more than 1/120 part of the whole.
The foregoing experiments show that the chief cause of the diminution which common air, or a mixture of common and dephlogisticated air, suffers by the electric spark, is the conversion of the air into nitrous acid; but yet it seemed not unlikely, that when any liquor, containing inflammable matter, was in contact with the air in the tube, some of this matter might be burnt by the spark, and thereby diminish the air, as I supposed in the above-mentioned paper to be the case. The best way which occurred to me of discovering whether this happened or not, was to pass the spark through dephlogisticated air, included between different liquors; for then, if the diminution proceeded solely from the conversion of air into nitrous acid, it is plain that, when the dephlogisticated air was perfectly pure, no diminution would take place; but when it contained any phlogisticated air, all this phlogisticated air, joined to as much of the dephlogisticated air as must unite to it in order to reduce it into acid, that is, 2 or 3 times its bulk, would disappear, and no more; so that the whole diminution could not exceed 3 or 4 times the bulk of the phlogisticated air: whereas, if the diminution proceeded from the burning of the inflammable matter, the purer the dephlogisticated air was, the greater and quicker would be the diminution. The result of the experiments was, that when dephlogisticated air, containing only 1/20 of its bulk of phlogisticated air, that being the purest air I then had, was confined between short columns of soap-lees, and the spark passed through it till no further diminution could be perceived, the air lost 43/200 of its bulk; which is not a greater diminution than might very likely proceed from the first-mentioned cause; as the dephlogisticated air might easily be mixed with a little common air while introducing into the tube.
When the same dephlogisticated air was confined between columns of distilled water, the diminution was rather greater than before, and a white powder was formed on the surface of the quicksilver beneath; the reason of which probably was, that the acid produced in the operation corroded the quicksilver, and formed the white powder; and that the nitrous air, produced by that corrosion, united to the dephlogisticated air, and caused a greater diminution than would otherwise have taken place. When a solution of litmus was used, instead of distilled water, the solution soon acquired a red colour, which became paler and paler as the spark was continued. When lime-water was let up into the tube, a cloud was formed, and the air was further diminished by about 1/5. The remaining air was good dephlogisticated air. In this experiment therefore the litmus was, if not burnt, at least decompounded, so as to lose entirely its purple colour, and to yield fixed air; so that, though soap-lees cannot be decompounded by the process, yet the solution of litmus can, and so very likely might the solutions of many other combustible substances. But there is nothing, in any of these experiments, which favours the opinion of the air being at all diminished by means of phlogiston communicated to it by the electric spark.
[1] From what follows it appears, that the reason why the air ceased to diminish was, that as the soap-lees were then become neutralized, no alkali remained to absorb the acid formed by the operation, and in consequence scarce any air was turned into acid. The spark however was not continued long enough after the apparent cessation of diminution, to determine with certainty, whether it was only that the diminution went on remarkably slower than before, or that it was almost come to a stand, and could not have been carried much further, though I had persisted in passing the sparks.
[2] This air was as pure as any that can be procured by most processes. I propose giving an account of the experiment, in which it was prepared, in a future paper.
 Back to the list of selected historical papers.
Back to the list of selected historical papers.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.
 The apparatus used in making the experiments was as follows: The air through which the spark was intended to be passed, was confined in a glass tube M, bent to an angle, as in fig. 4, pl. 1, which, after being filled with quicksilver, was inverted into two glasses of the same fluid, as in the figure. The air to be tried was then introduced by means of a small tube, such as is used for thermometers, bent in the manner represented by ABC, fig. 5, the bent end of which, after being previously filled with quicksilver, was introduced, as in the figure, under the glass DEF, inverted into water, and filled with the proper kind of air, the end C of the tube being kept stopped by the finger: then, on removing the finger from C, the quicksilver in the tube descended in the leg BC, and its place was supplied with air from the glass DEF. Having thus got the proper quantity of air into the tube ABC, it was held with the end C uppermost, and stopped with the finger; and the end A, made smaller for that purpose, being introduced into one end of the bent tube M, fig. 4, the air, on removing the finger from C, was forced into that tube by the pressure of the quicksilver in the leg BC. By these means I was enabled to introduce the exact quantity I pleased of any quantity of soap-lees, or any other liquor which I wanted to be in contact with the air.
The apparatus used in making the experiments was as follows: The air through which the spark was intended to be passed, was confined in a glass tube M, bent to an angle, as in fig. 4, pl. 1, which, after being filled with quicksilver, was inverted into two glasses of the same fluid, as in the figure. The air to be tried was then introduced by means of a small tube, such as is used for thermometers, bent in the manner represented by ABC, fig. 5, the bent end of which, after being previously filled with quicksilver, was introduced, as in the figure, under the glass DEF, inverted into water, and filled with the proper kind of air, the end C of the tube being kept stopped by the finger: then, on removing the finger from C, the quicksilver in the tube descended in the leg BC, and its place was supplied with air from the glass DEF. Having thus got the proper quantity of air into the tube ABC, it was held with the end C uppermost, and stopped with the finger; and the end A, made smaller for that purpose, being introduced into one end of the bent tube M, fig. 4, the air, on removing the finger from C, was forced into that tube by the pressure of the quicksilver in the leg BC. By these means I was enabled to introduce the exact quantity I pleased of any quantity of soap-lees, or any other liquor which I wanted to be in contact with the air.

 Back to the list of selected historical papers.
Back to the list of selected historical papers.