Charles-Adolphe Wurtz (1817-1884)
Account of the Sessions of the International Congress of Chemists in Karlsruhe, on 3, 4, and 5 September 1860[1]
originally published in Richard Anschütz, August Kekulé, 2 vols. (Berlin: Verlag Chemie, 1929) as Appendix VIII (pp. 671-88 of vol. 1); English translation by John Greenberg and William Clark published in Mary Jo Nye, The Question of the Atom (Los Angeles: Tomash, 1984)]
It was Mr. Kekulé's idea to bring about an international meeting of chemists. During the fall of 1859 he had an opportunity to make the initial overtures in this regard--first to Mr. Weltzien, then to Mr. Wurtz. At the end of March 1860, these three scientists, all in Paris at that moment, devised the initial steps to be taken in order to carry out the plan in question. An initial circular was composed, which had as its aim winning the support of the most outstanding men of the science. It noted, in general terms, the differences that had arisen between the theoretical views of chemists and the urgency of putting an end to these differences by a common agreement, at least where certain questions were concerned.
The first appeal having been favorably received, an understanding was reached on the time and place of the meeting, and printing of a second circular addressed to all European chemists, which explained the objectives and goals of an international congress in the following terms was agreed upon:[2]
Paris, 15 June 1860
Dear Distinguished Colleague,
The great development that has taken place in chemistry in recent years, and the differences in theoretical opinions that have emerged, make a Congress, whose goal is the discussion of some important questions as seen from the standpoint of the future progress of the science, both timely and useful.
The undersigned invite to this meeting all chemists authorized by their work or position to express an opinion in a scientific discussion.
Such an assembly cannot deliberate on behalf of everyone, nor can it pass resolutions by which everyone must abide, but by means of a free and thorough discussion, certain misunderstandings could be eliminated, and a common agreement facilitated on some of the following points: the definition of important chemical notions, such as those expressed by the words atom, molecule, equivalent, atomic, basic; the examination of the question of equivalents and of chemical formulae; the institution of a notation and of a uniform nomenclature.
Knowing that the assembly's deliberations would not be of a nature such as to reconcile all opinions and eliminate all disagreements immediately, the undersigned believe, nevertheless, that such works could pave the way for a much desired agreement between chemists in the future, at least where the most important questions are concerned. A commission could be charged to continue the investigation of these questions and to interest in them learned academies or societies with the necessary material means for resolving them.
The Congress will convene in Karlsruhe on 3 September 1860.
Our colleague, Mr. Weltzien, Professor at the Polytechnic School in this city, wishes to take on the duties of General Commissioner. In this capacity, he will be in charge of registering prospective members for the Congress and will open the assembly at nine o'clock in the morning on the day indicated.
In conclusion, and with the aim of avoiding any unfortunate omissions, the undersigned request that the individuals to whom this circular will be sent please communicate it to their scientist friends who are duly authorized to attend the planned conference.
| Babo de, Freiburg | Fremy, Paris | Pelouze, Paris
|
| Balard, Paris | Fritzsche, St. Petersburg | Piria, Turin
|
| Bekétoff, Kasan | Hofmann, A. W., London | Regnault, V., Paris
|
| Boussingault, Paris | Kekulé, Ghent | Roscoe, Manchester
|
| Brodie, Oxford | Kopp, H., Geissen | Schroetter, A., Vienna
|
| Bunsen, Heidelberg | Hlasiwetz, Innsbruck | Socoloff, St. Petersburg
|
| Bussy, Paris | Liebig, J. de, Munich | Staedler, Zurich
|
| Cahours, Paris | Malaguti, Rennes | Stas, Brussels
|
| Cannizzaro, Genoa | Marignac, Geneva | Strecker, Tübingen
|
| Deville, H., Paris | Mitscherlich, Berlin | Weltzien, C., Karlsruhe
|
| Dumas, Paris | Odling, London | Will, H., Giessen
|
| Engelhardt, St. Petersburg | Pasteur, Paris | Williamson, W., London
|
| Erdmann, O. L., Leipzig | Payen, Paris | Wöhler, F., Göttingen
|
| Fehling de, Stuttgart | Pebal, Vienna | Wurtz, Ad., Paris
|
| Frankland, London | Peligot, Paris | Zinin, St. Petersburg
|
Nota Bene: You can sign up for the conference either directly with Mr. Weltzien, Polytechnic School, Karlsruhe, or with Mr. A. Kekulé, Professor of Chemistry at the University of Ghent, who will pass it on to Mr. Weltzien.
The number of people who wanted to participate was considerable, and on 3 September 1860, 140 chemists[3] met together in the meeting room of the second Chamber of State, which was made available by the Archduke of Baden.
The list of the chemists in attendance follows:[4]
- BELGIUM. Brussels: Stas; Ghent: Donny, A. Kekulé.
- GERMANY. Berlin: Ad. Baeyer, G. Quinke; Bonn: Landolt; Breslau: Lothar Meyer; Kassel: Guckelberger; Klausthal: Streng; Darmstadt: E. Winkler; Erlangen: v. Gorup-Besanez; Freiburg i.B.: v. Babo, Schneyder; Giessen: Boeckmann, H. Kopp, H. Will; Göttingen: F. Beilstein; Halle a.S.: W. Heintz; Hanover: Heeren; Heidelberg: Becker, O. Braun, R. Bunsen, L. Carius, E. Erlenmeyer, O. Mendius, Schiel; Jena: Lehmann, H. Ludwig; Karlsruhe: A. Klemm, R. Muller, J. Nessler, Petersen, K. Seubert, Weltzien; Leipzig: O. L. Erdmann, Hirzel, Knop, Kuhn; Mannheim: Gundelach, Schroeder; Marburg a.L.: R. Schmidt, Zwenger; Munich: Geiger; Nuremberg: v. Bibra; Offenbach: Grimm; Rappenau: Finck; Schönberg: R. Hoffmann; Speyer: Keller, Mühlhaüser; Stuttgart: v. Fehling, W. Hallwachs; Tübingen: Finckh, A. Naumann, A. Strecker; Wiesbaden: Kasselmann, R. Fresenius, C. Neubauer; Würzburg: Scherer, v. Schwarzenbach.
- ENGLAND. Dublin: Apjohn; Edinburgh: Al. Crum Brown, Wanklyn, F. Guthrie; Glasgow: Anderson; London: B. J. Duppa, G. C. Foster, Gladstone, Müller, Noad, A. Normandy, Odling; Manchester: Roscoë; Oxford: Daubeny, G. Griffeth, F. Schickendantz; Woolwich: Abel.
- FRANCE. Montpellier: A. Béchamp, A. Gautier, C. G. Reichauer; Mülhousen i.E.: Th. Schneider; Nancy: J. Nicklès; Paris: Boussingault, Dumas, C. Friedel, L. Grandeau, Le Canu, Persoz, Alf. Riche, P. Thénard, Verdét, Wurtz; Strasbourg i.E.: Jacquemin, Oppermann, F. Schlagdenhaussen, Schützenberger; Tann: Ch. Kestner, Scheurer-Kestner.
- ITALY. Genoa: Cannizzaro; Pavia: Pavesi.
- MEXICO. Posselt.
- AUSTRIA. Innsbruck: Hlasiwetz; Lemberg: Pebal; Pesth: Th. Wertheim; Vienna: V. v. Lang, A. Lieben, Folwarezny, F. Schneider.
- PORTUGAL. Coïmbra: Mide Carvalho.
- RUSSIA. Kharkov: Sawitsch; St. Petersburg: Borodin, Mendelyeev; L. Schischkoff, Zinin; Warsaw: T. Lesinski, J. Natanson.
- SWEDEN. Harpenden: J. H. Gilbert; Lund: Berlin, C. W. Blomstrand; Stockholm: Bahr.
- SWITZERLAND. Bern: C. Brunner, H. Schiff; Geneva: C. Marignac; Lausanne: Bischoff; Reichenau bei Chur: A. v. Planta; Zurich: J. Wislicenus.
- SPAIN. Madrid: R. de Suna.
First Session of the Congress
Mr. Weltzien, General Commissioner, opened the first session with the following speech:
Gentlemen:
As provisional chairman, I have the honor to inaugurate a Congress which has no precedent for its kind, the nature of which has never before met. To be sure, German Natural Scientists and Physicians, upon the instigation of Oken, and emulating their Swiss Colleagues, have assembled almost annually for scientific conferences in various cities of the Fatherland since 1822. Following the lead of such congresses, English, French, and during the past several years, also Scandinavian Natural Scientists have convened for a similar purpose. Devotees of the different branches of Natural Science and Medicine were regularly present, although all participants were invariably of the same nationality. The business of these congresses was for the most part characterized by reports, the topics of which were not integrated into any previously arranged program, but rather, as presentations of work in progress, left to the discretion of each individual. A lively and amicable intercourse, flavored by a sequence of festivities, united the ethnically and linguistically related Natural Scientists and Physicians for several days.
Not so for the Congress convened here today. For the first time, the representatives of a single, and indeed the newest Natural Science have assembled. These representatives belong, however, to nearly every nationality. We may be of differing ethnic origin and speak different languages, but we are related by professional specialty, are bound by scientific interest, and are united by the same design. We are assembled for the specific goal of attempting to initiate unification around points of vital concern for our beautiful science. Due to the extraordinarily swift development of Chemistry, and especially because of the massive accumulation of factual materials, the theoretical standpoints of researchers and the means of expression, both in words and symbols, have begun to diverge more than is expedient for mutual understanding, and, especially, more than is suitable for instruction. Considering the importance of Chemistry for other natural Sciences and its indispensability for technology, it seems exceedingly desirable and advisable to cast our science in a more rigorous form, so that it will be possible to communicate it in a relatively more concise manner.
In order to achieve this, we should not be constrained to only review various viewpoints and writing conventions, the variety of which offers little of importance; and we should not be burdened with a nomenclature, which in view of a plethora of unnecessary symbols lacks any rational basis, and which, making matters worse, is derived, for the most part, from a theory whose validity can hardly be maintained today. The ample attendance at this Congress is surely a clear indication that these nuisances are universally recognized and that their removal from the path toward unification appears desirable. The achievement of this end is a prize of such beauty that it is well worth the effort to undertake the task here.
The original notion of a Chemistry-Congress was communicated to me some time ago by our colleague Kekulé. Early this year, I initiated the first steps toward its realization. The ripeness of this undertaking was manifoldly acknowledged; I obtained unsolicited support from all quarters. Because of this, I do not doubt that this Congress will be called upon to lay the foundations for a not unimportant epoch in the history of our science.
The city of Karlsruhe, which two years ago had the great fortune to host one of the most splendid congresses of German Natural Scientists and Physicians, has now the honor of seeing the first international Chemistry-Convention within its city walls. Karlsruhe is the capital of a small but blessed province, in which, under the auspices of a noble prince and a liberal government, the Arts and Sciences flourish, and where its devotees, esteemed and sustained, can follow their calling with devotion and good cheer. Since it is my pleasure to bid you a hearty welcome to this city, I expect that the same good cheer will permeate our Congress, and hope that our science will one day look back with satisfaction upon our assembly.
After this speech the general commissioner first asks Mr. Bunsen to preside, but the latter refuses and asks the assembly to encourage Mr. Weltzien to direct the deliberations during the first session. Mr. Weltzien is named President. Messrs. Wurtz, Strecker, Kekulé, Odling, Roscoe, and Schischkoff are appointed to serve as Secretaries.
At the suggestion of Mr. Kekulé, the assembly decides that a commission will be put in charge of drawing up a list of questions that will be submitted to the Congress for deliberation.
Mr. Kekulé takes the floor in order to explain the schedule of questions. (Then an analysis of Mr. Kekulé's speech follows.[5])
Mr. Erdmann emphasizes the necessity of directing the assembly's deliberations and resolutions towards questions of form, rather than doctrinal points.
A discussion begins over the question of whether the Commission will hold its sessions behind closed doors or during plenary sessions of the entire assembly.
After some words among Messrs. Fresenius, Kekulé, Wurtz, Boussingault, and H. Kopp, the assembly decides, upon the motion made by the last individual named, that the sessions will take place behind closed doors.
First Session of the Commission
The commission met on 3 September at 11 A.M., Mr. H. Kopp presiding.
The chairman suggests that the discussion begin with the notions of molecule and atom, and he asks Mr. Kekulé and Mr. Cannizzaro, whose studies have especially encompassed this issue, to take the floor.
Mr. Kekulé emphasizes the need to distinguish between the molecule and atom, and, in principle at least, the physical molecule and the chemical molecule.
Mr. Cannizzaro is unable to conceive of the notion of the chemical molecule. For him there are only physical molecules, and the Ampère-Avogadro law is the basis for considerations relating to the chemical molecule. The latter is nothing other than the gaseous molecule.
Mr. Kekulé thinks, on the contrary, that the chemical facts must serve as the basis for the definition and determination of the (chemical) molecule and that physical considerations should only be invoked as a check.
Mr. Strecker points out that in certain cases the atom and molecule are identical, as in the case of ethylene.
Mr. Wurtz says that a certain difficulty can be sensed in defining the chemical molecule of oxygen and the diatomic elements in general, which are comparable to ethylene. The view of these as molecules formed of two atoms derived from physical considerations[6] but until now no chemical fact appears to militate in favor of this doubling.
Mr. H. Kopp, summarizing the discussion, says that the need to separate the idea of the molecule from that of the atom appears to be established; that the notion of the molecule can be fixed with the help of purely chemical considerations; that the definition does not have to involve density alone; and, finally, that it appears natural to call the largest quantity the molecule, and the smallest quantity the atom. In concluding, the speaker formulates the first question to be put to the assembly. This question is as follows: "Is it appropriate to establish a distinction between the terms molecule and atom, and to call molecules, which are comparable as far as physical properties go, the smallest quantities of bodies which enter into or come out of a reaction, and to call atoms the smallest quantities of bodies which are contained in these molecules?"
Mr. Fresenius calls attention to the expression compound atom and said that these two words entail a contradiction. Mr. Fresenius's remark motivates the drawing up of a second question to be submitted to the assembly, which is as follows: "Can the expression compound atom be eliminated and replaced by the expressions radical or residue?"
Mr. Kopp goes back to the program explained by Mr. Kekulé, and he calls attention to the definition of the word equivalent. It seems to him that the notion of equivalent is perfectly clear and is sharply distinguished from the notion of molecule and that of atom. Consequently, the commission adopts, without discussion, the third proposition to be submitted to the assembly, which is as follows: "The notion of equivalents is empirical and independent of the idea of molecule and that of atom."
The session continues, with Mr. Erdmann presiding.[7] The discussion on notation gets underway: Mr. Kekulé points out that molecular and atomic notation, or the notation of equivalents, can be employed, but that as in any system it is necessary to stick rigorously to the particular notation, whichever it be, once adopted.
The meaning of the word "equivalent" is the object of several remarks. Mr. Béchamps says that equivalence can only be assumed in cases where the functions of bodies are identical.
Mr. Schischkoff is not of the same opinion. He thinks that the notation for equivalence and equivalent quantities is independent of chemical functions. Everyone assumes an equivalence between chlorine and hydrogen. After a few observations presented by other members on the same subject, the session was adjourned.
Second Session of the Congress
Mr. Boussingault presiding
In taking the chair, Mr. Boussingault thanks the Congress for having bestowed the honor of presiding upon a scientist whose studies have had matters of applied chemistry as their object, rather than points of abstract theory. The chairman sees in this choice that the Congress wanted to testify to the unity of the so-called old and new chemistries. He protests against these terms, and remarks that it is not chemistry that grows old, but chemists.
The chairman announces that the work of the Commission is not ready, but that it has agreed upon the drawing up of three questions to be submitted to the assembly for deliberation. He asks one of the Secretaries to make these known to the assembly.
Mr. Strecker takes the floor and reads to the assembly the questions drafted by the commission and indicated above.
Mr. Kekulé enlarges upon the points specified in the first question. Concerning the fundamental hypothesis which can be made about the nature of matter, the speaker wonders if it is necessary to adopt the atomic hypothesis or if a dynamical hypothesis is enough. The first alternative seems preferable to him. Dalton's hypothesis was verified by everything known about the nature of gases. One is authorized to assume small units or small components in gases, and when the same body can affect the gaseous state, the solid state, and the crystalline state, it is possible that the crystalline molecules are precisely the small gaseous components in question, or that these are a fraction of others. But the nature of these relations cannot be specified. What is certain is that in chemical reactions there exists a quantity that enters into or comes out of reaction in the smallest proportion, and never as a fraction of this proportion. These quantities are the smallest that can exist in a free state. These are the molecules defined chemically. But these quantities are not indivisible; chemical reactions succeed in cutting them and resolving them into absolutely indivisible particles. These particles are atoms. The elements themselves, when they are free, consist of molecules formed of atoms.[8] Thus the free chlorine molecule is formed from two atoms. This leads one to assume different molecular and atomic units:
- physical molecules
- chemical molecules
- atoms
The gaseous physical molecules have not been shown to be identical with the physical molecules of solids and liquids. Secondly, the chemical molecules have not been shown to be identical with gaseous molecules. Thus it is not established if the smallest quantity of a substance that enters into a reaction is also the smallest quantity of this substance that plays a role in heat phenomena.
It must be said, however, that the chemical molecule is normally identical to the physical molecule. It has even been maintained that the first never represents anything more than the second. For the speaker it is not like that. The chemical molecule has an independent existence, and in order to allow the distinction in question to be assumed, it is enough to demonstrate its reality in a few cases. But that is easy. Has it not been shown that, for the density of sulphur vapor, the chemical molecules do not always completely separate from one another, but remain fused together in certain conditions (at 500°) to form physical molecules?
The speaker adds that the existence and magnitude of chemical molecules can and must be determined by chemical demonstrations and that the physical facts are not enough to achieve this result.[9] With the help of physical considerations, how could it be shown that hydrochloric acid is formed of a single hydrogen atom and a single chlorine atom? Would it not be enough to multiply the formula HCl by a certain coefficient, and to do the same for all other formulae, in order to establish a perfect agreement between physical properties?
Mr. Cannizzaro takes the floor in order to point out that the distinction between physical and chemical molecules appears to him neither to be necessary nor clearly established.
Mr. Wurtz expresses the opinion that this is a secondary point and can be reserved. It seems to him, on the contrary, that the question relative to establishing the distinction between the terms "molecule" and "atom" has nearly been concluded, and that everyone seems to recognize the utility of such a distinction. It is a matter of clarifying the sense of words generally in common use; it is purely a matter of a definition, and the speaker thinks that in a question of this type, there would perhaps be propriety and usefulness in the expression, by the assembly, of an opinion following the discussion. This opinion, moreover, would not bind anyone, and there would be nothing obligatory about it.
Discussion of the second question relative to the words "compound radical" begins. Mr. Miller thinks that scientific language could not do without the words "compound atom." There are atoms of simple substances; there are atoms of compound substances.
Messrs. Kekulé, Natanson, Strecker, Ramon de Luna, Nicklès, Béchamps and other members present varied observations in one direction or another, but the discussion of this question, like that of the preceding one, leads to no resolution from the assembly.
Second Session of the Commission
Mr. H. Kopp presiding
Mr. Kekulé explains his ideas on chemical notation. He points out that either an atomic-molecular notation or a notation in equivalents can be employed. In the first case, the chemical formula represents the molecule; in the second, it represents equivalence. The following examples illustrate this distinction:
| atomic molecular notation || | notation in equivalents
|
| H Cl | H Cl
|
H2O | HO
|
| H3Az | H az[10] |
The important thing is not to mix these notations and get them confused, as is often done. They get mixed up if water is written HO = 9 and ammonia, H3Az = 17, etc.
Mr. Cannizzaro stresses the importance of considerations relating to volumes in the question of notation. The arguments elaborated by the speaker are reproduced in extenso in the report of the third session of the Congress (see ahead).
The chairman draws attention to the overly detailed course of the discussion, and he points out that, within the Commission, the questions should be indicated rather than investigated. He also deems that the discussion relative to notation in equivalents, such as had just been formulated by Mr. Kekulé, can be left aside. No one is served by it. The speaker believes that it would be proper not to get too attached to theoretical matters concerning the content of things, and to stick to questions of form. Several members express a similar opinion, and Mr. Erdmann, in particular, draws attention to the urgency of adopting a notation whose symbols always represent one and the same given value.
Summarizing the discussion, the chairman acknowledges that given the recent advances in the science, it is likely that certain atomic weights ought to be doubled, but that it would be useful to take into consideration the notation that has, until now, generally been employed in introducing notation to represent these double weights and not to adhere too rigorously to the symbols in the latest notation representing different values. As a transitional measure and to avoid confusion, he thinks it convenient to adopt certain signs to indicate the differences in question. Consequently, the chairman approves of the habit of some chemists, that of barring double atomic weights. In concluding, he formulates the question to be submitted before the Congress in the following manner:
"Is it desirable to harmonize chemical notation with recent advances in the science by doubling a certain number of atomic weights?"
Third Session of the Commission
Mr. Dumas presiding
Mr. Kekulé summaries the discussion of the preceding session and repeats the question, announced by Mr. Kopp, in a slightly mitigated form. According to the speaker, this question should be posed in the following way:
"Do the recent advances in science warrant a change in notation?"
Mr. Strecker proposes that atomic notation be adopted in principle.
The chairman stresses forcefully the disadvantages that result from current confusion. He points out that this state of affairs, were it to continue, would be such as to undermine not only the proper direction of teaching and advances in science, but the reliability of industrial work as well. Let us think back, says the chairman, to what we remember from twenty years earlier. Berzelius's table of atomic weights was both the underlying support for the whole science of chemistry and the infallible guide to industrial operations. There is nothing today to replace this universally acknowledged authority, and we have to be careful that chemistry does not fall from the high rank that it has enjoyed among the sciences until now.
Mr. Wurtz is pleased to acknowledge that Mr. Dumas has gotten to the core of the issue, and he thinks it necessary to return to the principles of atomic weights and to Berzelius's notation. According to the speaker, marginal changes in the interpretation of some facts would suffice to bring the principles and this notation into harmony with the requirements of modern science. The notation suitable for adoption today is not exactly Gerhardt's. Gerhardt rendered enormous services to the science. Today he is dead, and his name, the speaker says, should only be spoken with respect. However, it seems that this chemist made two mistakes. One concerns form alone, the other is inherent in the root of things.
First, instead of presenting his notation as founded upon new principles, he more moderately linked it to Berzelius's principles, thus sheltering his innovation under the authority of this great name. Secondly, it seems that Gerhardt made a mistake in likening all of the oxides of inorganic chemistry to silver oxide and to anhydrous potassium oxide, and in attributing to them, as in the case of the latter, the formula 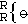 . In organic chemistry there should be oxides corresponding to ethylene oxide, just as there are representatives of ethyl oxide and of others that correspond to glycerol oxide, and if potassium hydrate, for example, can be compared to alcohol, then other hydrates should be compared with glycol and glycerine. It is understandable that these considerations are such as to prompt and justify some changes in Gerhardt's notation and in the atomic weights that he attributed to certain metals.
. In organic chemistry there should be oxides corresponding to ethylene oxide, just as there are representatives of ethyl oxide and of others that correspond to glycerol oxide, and if potassium hydrate, for example, can be compared to alcohol, then other hydrates should be compared with glycol and glycerine. It is understandable that these considerations are such as to prompt and justify some changes in Gerhardt's notation and in the atomic weights that he attributed to certain metals.
After a discussion in which Messrs. Cannizzaro, Wurtz, and Kekulé take part, Mr. Kekulé opines that the question is well enough prepared to submit to the Congress for deliberation, and he asks that the writing of this question be entrusted to the Secretaries.
This proposition is adopted by the Commission.
Third Session of the Congress
Mr. Dumas presiding
In taking the chair, the chairman addresses some words of thanks to the assembly and expresses the hope that a common agreement on some of the questions aired before the Congress will be reached.
Next, the Chairman suggests to the assembly that two Vice-chairmen be designated. Messrs. Will and Miller are appointed to these positions. Mr. Odling replaces Mr. Roscoe, who had to leave, as Secretary. Next, the Secretaries read the questions, whose writing had been entrusted to hem, and which have been worked out by the Commission. These questions are conceived as follows:
"Is it desirable to harmonize chemical notation with advances in the science?"
"Is it appropriate to adopt the principles of Berzelius again, where notation is concerned, in bringing about some modifications to these principles?"
"Is it desirable to distinguish new chemical symbols from those which were generally in use fifteen years ago with the help of particular signs?"
Mr. Cannizzaro takes the floor in order to oppose the second proposition. It scarcely appears fitting or logical to him to move science back to the time of Berzelius, so as to make chemistry again cover the path that it has already taken. In effect, Berzelius's system has already undergone successive modifications, and these modifications have led to Gerhardt's system of formulae. And these changes were not at all introduced abruptly or without transitions into the system or without transitions into the system; they were the result of successive advances. If Gerhardt had not proposed them, Mr. Williamson or Mr. Odling, or another chemist who had taken part in the evolution of science, would have done so.
"The source that Gerhardt's system goes back to is the Avogadro-Ampère theory of the uniform constitution of substances in the gaseous state. This theory leads us to view the molecules of certain simple substances as susceptible to division in the future. Mr. Dumas understood the importance of Avogadro's theory and all of its consequences. He posed this question: Is there agreement between the results of Avogadro's theory and the results deduced by means of other methods used to determine the relative weights of molecules? Realizing that science was still short of experimental results of this kind, he wanted to gather together the largest possible number before risking any general conclusion on this subject. Thus he got down to work, and with the help of the method which he applied to determine vapor densities, he furnished science with valuable results. However, it appears that he never got far enough along with the method to be able to infer from the results acquired the general conclusion for which he aimed. Be that as it may concerning this reserve he thought necessary, it can be said that it was he who put chemists on the path to Avogadro's theory, because he, more than anyone else, was responsible for introducing the habit of choosing formulae for volatile substances corresponding to the same volume as that taken up by hydrochloric acid and ammonia.
"The most evident display of this influence of Mr. Dumas's school appears in a paper by one of his students, Mr. Gaudin. Mr. Gaudin accepted Avogadro's theory without reservation. He established a clear-cut distinction between the words atom and molecule, by means of which he was able to reconcile all facts with theory. This distinction had already been made by Mr. Dumas, who had called the molecule the physical atom in his lessons on chemical philosophy. It is certainly a mainspring of Gerhardt's system.
"Sticking more closely to Avogadro's theory than Gerhardt did later, and taking advantage of new experimental data on vapor densities, Mr. Gaudin established that atoms are not always the same fraction of the molecules of simple bodies--that is to say, these molecules do not always result from the same number of atoms; while the molecules of oxygen, hydrogen, and other halogens are formed from two atoms, the molecule of mercury is made of a single atom. He went so far as to compare the composition of equal volumes of alcohol and ether in order to deduce the relative composition of their molecules. But his mind did not seize upon all of the results of this comparison, and chemists have forgotten the idea that he had. And yet this comparison was one of the starting points for Gerhardt's proposed reform.
"Other chemists, Proust among them, also accepted Avogadro's theory and arrived at the same general conclusions as Mr. Gaudin.
"What did Gerhardt do in this state of the science?
"He accepted Avogadro's theory and the consequence that atoms of simple bodies are divisible, and he applied this theory to deduce the relative make-up of the molecules of hydrogen, oxygen, chlorine, nitrogen, hydrochloric acid, water, and ammonia. If he had stopped there, he would not have gotten ahead of Avogadro and Mr. Dumas. But he then subjected all of the formulae of organic chemistry to a general investigation, and he realized that all of these formulae corresponding to equal volumes of hydrochloric acid and ammonia were confirmed by all reactions and by all chemical analogies. Thus he contemplated modifying formulae that were the exception to the rule introduced by Mr. Dumas. He tried to show that the reasons for the violation of the equal volumes rule were unfounded. To reduce the formulae of all volatile substance of organic chemistry to equal volumes had been the starting point for Gerhardt's proposed reforms. The modifications of atomic weights of certain simple substances, the discovery of the relations that the hydrates, whether acidic or basic, have with water, had been the consequence of this first step. What happened next? The unforgettable experiments of Mr. Williamson on etherification, on mixed ethers, on acetones, those of Gerhardt on anhydrous acids, those of Mr. Wurtz on alcoholic radicals, etc., successively confirmed what Gerhardt had predicted as a consequence of his system. Thus there occurred in chemistry something analogous to what happened in optics when the undulatory theory was introduced. This theory predicted with wonderful accuracy the facts that experiments later confirmed. Gerhardt's system in chemistry was not less fruitful in exact predictions. It is intimately mixed up with and tied to all of the works of chemistry which had preceded it and to all of the advances that followed it in the history of the science. It is not an abrupt leap, an isolated event. It is a regular step forward, small in appearance, but large in results. From now on, this system cannot be effaced from the history of science. It can and must be discussed and modified. But it is the system that must be taken as the starting point, when it is a matter of introducing into chemical science a system of formulae in accord with the actual state of our knowledge. Some chemists will perhaps be tempted to say: the difference between Gerhardt's formulae and those of Berzelius is very small, because the formula for water, for example, is the same in the two systems. But we must be careful. The difference is very small in appearance, but it is large at bottom. Berzelius was under the influence of Dalton's ideas. The idea of a difference between the atom and the molecule of substances never entered his mind. In all of his arguments he assumed implicitly that atoms of simple substances, are, vis-à-vis physical forces, units of the same order, compound atoms. For this reason he began by assuming that equal volumes contain the same number of atoms. Soon he realized that this rule could only be applied to simple substances, and throughout the whole of his scientific career, he attributed no value to atoms of compound substances in choosing formulae. He was even forced to restrict the rule for equal numbers of atoms in equal volumes to a very small number of simple substances--that is, to those that are permanent gases--thereby introducing into the makeup of gases and vapors a difference that no physicist was ever able to admit. Berzelius did not assume that molecules of simple substances could divide in combining. On the contrary, he assumed that two molecules often form the quantity that enters wholly into the combination. This is what he called double atoms. Thus he assumed that water and hydrochloric acid contain the same quantity of hydrogen--a quantity equal to two physical molecules joined together.
"So you see, gentlemen, what a profound difference exists between the ideas of Berzelius and those of Avogadro, Ampère, Mr. Dumas, and Gerhardt.
"I am surprised that Mr. Kekulé, who said in his book that Gerhardt is the first and only one who completely understood the atomic theory, has accepted the commission's proposition.
"I believe that I have shown, Mr. Cannizzaro continued, that a discussion of formulae must take as starting points, the formulas of Gerhardt, but I do not maintain that all of them must be accepted in the form that he proposed them. Far from it; I tried, some years ago, to introduce certain modifications into them, in such a way as to avoid the inconsistencies which appeared to me to exist in Gerhardt's system. In effect, it is strange to see how this chemist renounced Avogadro's theory after having used it as the basis for his reforms. Here is how he put it himself: 'There are molecules in 1, 2 and 4 volumes, as there are in 1/2, in 1/3, in 1/4 of a volume.' (Comptes rendus des travaux de Chimie, 1851, p. 146). And he continued as follows (p. 147): 'It is perhaps surprising to see me defend this thesis, when I have recommended and still recommend every day that a regular notation in organic chemistry be followed, in representing all volatile substances by the same number of volumes, by 2 or by 4. The chemists who see 2 contradictory assertions in that forget that I never acknowledged the preceding principle as a molecular truth, but as a condition to be satisfied in order to arrive at the knowledge of certain laws or certain relations that would be allowed to escape the observer's attention in an arbitrary notation, or one suitable for special cases.'
"There certainly were facts that forced Gerhardt to renounce Avogadro's theory, but there were also unwarranted hypotheses. The facts were the densities of monohydrate sulfuric acid vapor, of sal ammoniac, and of phosphorus perchloride.
"You already know, gentlemen, that on the occasion of the publication of the paper of Mr. Deville on the dissociation of certain compounds by heat, I was the first to try to interpret the occurrence of these abnormal densities, by supposing that the bodies in question are split in two, and that in reality a mixture of vapors is weighed in the determination of these densities. After me, Mr. H. Kopp proposed the same interpretation in his own way.
"I will not repeat here the arguments that we invoked in favor of this interpretation. I will only add that one of the members of this Congress just told me that the boiling point of sulphuric acid is almost constant at very different pressures--a fact that shows that it is not a matter of a boiling point here, but of a decomposition point. I am convinced that other facts will confirm the interpretation that we have given to abnormal densities, and, as a result, will dispel the doubts that some scientists still appear to harbor concerning Avogadro's theory.
"But independently of the facts that I have just cited, there were also unwarranted hypotheses that led Gerhardt away from Avogadro's theory. I am going to show that this is the case.
"Gerhardt took as a demonstrated truth that all metallic compounds have formulae analogous to those of the corresponding hydrogen compounds. From that it follows that the formulae for the mercury chlorides are HCl, Hg2Cl, in assuming that the free mercury molecule is formed of two atoms, like that of hydrogen. Let us observe that the vapor densities lead to a different result. In effect, in order to represent the composition of equal volumes of the following five bodies: hydrogen, hydrochloric acid, mercury, mercurous chloride, and mercuric chloride, we will have the following formulae:
H2, HCl, Hg2, Hg2Cl, Hg2Cl2
The comparison of these formulae show that in the molecules of free mercury and of its two chlorides, there exists the same amount of mercury, expressed by Hg2, and that mercurous chloride is analogous to hydrochloric acid, while the mercuric chloride contains twice the amount of chlorine in its molecule.
"As a result, the same reason that directed us to double the carbon atom also commits us to double the mercury atom. This comes down to saying that the amount of mercury expressed by Hg2 in the preceding formulae represents a single atom. In this case it is seen that the atom is equal to the molecule of the free body; and that in mercurous salts this atom is the equivalent of a single hydrogen atom, while in the mercuric salts it is the equivalent of two hydrogen atoms. In other terms, to employ the language generally in common use today, the mercury is monoatomic in the mercurous salts, but in the mercuric salts it is diatomic like the radicals of Mr. Wurtz's glycols.
"It is important to point out now that in doubling the atomic weight of mercury, as was done with the atomic weight of sulphur, we arrive at numbers that accord with the law of specific heats.
"But if the atomic weight of mercury is doubled, one is led by analogy to double those of copper, zinc, lead, tin, etc.--in a word, one ends up back in Mr. Regnault's system of atomic weights that agree with specific heats, with isomorphism, and with chemical analogies.
"That Gerhardt's system conflicted with the law of specific heats, as well as with isomorphism, was the truly unfortunate thing. This clash has produced two different chemistries--one, which dealt with inorganic substances, and accorded great value to isomorphism; and the other, which investigated organic substances, that took no account f this. Therefore, the same substance could not have the same formula in one chemistry as in the other. The clash that I have just indicated stemmed from the fact that Gerhardt's system was not entirely consistent; it disappears as soon as the inconsistencies are done away with.
"Vapor densities provide a means of determining the weight of molecules of substances, whether simple or compound. Specific heats are used to check the weights of atoms and not those of molecules. Isomorphism reveals analogies in molecular constitution.
"In support of the modification of atomic weights of certain metals which I have just suggested, I will cite the following facts: all of the volatile compounds of mercury, zinc, tin, and lead contain amounts of metal represented in ordinary notation by Hg2, Zn2, Sn2, Pb2. This fact alone is enough to indicate to us that these quantities represent the true atoms of the metals in question. The fact that there exist three oxalates of potassium and ammonium (monoatomic radicals), while there exist only two oxalates of barium and calcium (diatomic radicals), could also be cited. But for the moment, I do not stress this point, and I cannot deny, on the other hand, that there is one case where the atomic weight deduced from the comparison of molecular compositions is in conflict with the one deduced from specific heat. This case is relative to carbon. But it could be that the law of atomic heats remained masks by other causes that intervene in specific heat.
"In summary, gentlemen, I propose that Gerhardt's system be accepted, taking into consideration the modifications of the atomic weights of certain metals and the formulae for their salts which I suggest be brought about.
"And if we are unable to reach a complete agreement upon which to accept the basis for the new system, let us at least avoid issuing a contrary opinion that would serve no purpose, you can be sure. In effect, we can only obstruct Gerhardt's system from gaining advocates every day. It is already accepted by the majority of young chemists today who take the most active part in advances in science.
"In this case let us restrict ourselves to establishing some conventions for avoiding the confusion that results from using identical symbols that stand for different values. Generalizing already established custom, it is thus that we can adopt barred letters to represent the doubled atomic weights."
Mr. Strecker offers some clarifications concerning the drafting of the second proposition submitted to the Congress. This draft originally mentioned Gerhardt's name, but the majority of the committee had wanted to substitute Berzelius's name. The speaker did not share the opinion of the majority. It did not seem to him that there was good reason to go back to Berzelius, who could perhaps be criticized for a logical flaw on the question of atoms and equivalents. The useful and urgent thing is to improve what exists by taking into account the advances of science since Berzelius. Mr. Strecker adds that the doctrines expressed in "Gerhardt's system" offer real advantages. As for himself, he will henceforth adopt the new atomic weights in his papers, but he does not think that the time has come to introduce them into teaching and into elementary books.
Mr. Kekulé shares all of Mr. Cannizzaro's opinions. It appears useful to him, however, to have reservations about one point of detail. Mr. Cannizzaro considers mercurous chloride as containing HgCl (Hg=200). It appears more rational to Mr. Kekulé to envision it as a combination analogous to "Dutch liquid", that is, as containing Hg2Cl2 (Hg=200) and to assume that at the moment of vaporization the molecule Hg2Cl2 splits.[11]
Mr. Will does not wish to enter into the details of the questions submitted before the Congress. He confines himself to pointing out that the Congress must proceed directly to its goal. This goal is to find a clear, logical notation, incapable of generating confusion in the minds of those uninitiated in the formulae, and suitable for not only expressing the long accepted facts in science, but those that modern discoveries add to it each day as well.
Mr. Erdmann suggests that the first two questions be dropped and that discussion be confined to the last one. It appears difficult to him to reach an agreement concerning questions of principle, and especially to impose a notation by vote, as it were.
Mr. Wurtz points out that it was not anyone's idea to impose some idea or other. One is faced with two kinds of questions--those that concern the very root of things, and others that are questions of form. If there were not yet good grounds for resolving the first by vote, because they were not yet ripe enough, nothing prevents agreement on, and even voting on, the purely formal questions.
Mr. Hermann Kopp notes that, on many theoretical points, the opinions of chemists are divided. These differences of opinions are caused in part by misunderstandings and are reflected in the notation itself. A discussion could be very useful for terminating the misunderstandings.
Mr. Erlenmeyer suggests that barred symbols always be used to express atomic weights that represent the old double equivalents.[12]
Mr. L. Meyer points out that this point seemed settled, because no one has raised an objection in this respect.
A discussion among several members on the suitability of casting a vote began.
Mr. Cannizzaro's opinion is that it is pointless to vote on the third question.
Mr. Boussingault draws attention to the possible difficulty in misunderstanding the meaning of the votes the Congress can cast concerning the questions submitted before it. Voting is an expression of the wishes of the Congress and the Congress is not intending to impose the majority opinion on anyone.
Mr. Will aligns himself with the same opinion.
Mr. Normandy points out that the scientists who suggest the introduction of certain reforms concerning notation into science are those who principally cultivate organic chemistry. Now, it can be noted that the scientists do not even agree among themselves on some points. Thus it appears premature to apply principles which are still under discussion to inorganic chemistry.
Mr. Odling speaks about the question of barred symbols. He remembers that Berzelius introduced them into the science to express double atoms. The bar, he says, is thus the sign of divisibility, and it appears contrary to logic to bar symbols expressing indivisible atoms of oxygen and carbon.
Agreeing completely that Berzelius's double atoms had a different meaning from the indivisible atoms, the barring of whose symbols had been proposed, Mr. Kekulé points out that these barred symbols must express not the divisibility of atoms, but the divisibility of the value represented by these symbols, which is twice what they had been taken to be in the past.
In reply to the observations made by Messrs. Erdmann and Normandy, Mr. Kekulé adds that it is not enough to impose a theoretical opinion or a notation by vote, but that a discussion of such subjects is necessary and useful and will not fail to bear fruit.
The Congress consulted by the chairman expresses the wish that the use of barred symbols, representing atomic weights twice those that have been assumed in the past, be introduced into science.
Mr. Dumas adjourns the third and last session of the Congress, after paying respects to the Grand Duke of Baden and thanking him for his hospitality.
[1]I am indebted to my colleague, Prof. Gaufinez in Bonn, for examining the French text. [Note: I am reproducing all footnotes that appear in the 1929 publication. It is not clear to me which notes are Wurtz's and which Anschütz's. Some notes contain the parenthetical notation (A.); however, others, such as this one, also appear to be Anschütz's. --CJG]
[2]The circular was sent in German, French, and English. The German text is dated: "Carlsruhe, July 10, 1860"; the English text: "London, July 1, 1860." With the French version of the proceedings of the Congress, I have incorporated the French text of the circular, which, compared with the German and English, contains the name "Regnault" amongst the undersigned; the English lacks the name "Mitscherlich," as well.
[3]The printed list of members, supplemented by handwritten additions, contains 126 names. (A.)
[4]I have arranged the participants by country and by the cities in which they worked at the time. (A.)
[5]Cf. Appendix 9. (A.)
[6]In Kekulé's manuscript, he has added here the following marginal note: "Not always!" (A.)
[7]In Kekulé's manuscript, he has added: "Kekulé and Will declined."
[8]In Kekulé's manuscript, there is the following marginal note: "A molecule is 1, at most 2 atoms." (A.)
[9]The following note is in Kekulé's manuscript: "Striking example: NH4Cl, SO3.OH2." (A.)
[10]H = 1. C = 8. O = 16. Az = 14. az = 14/3.
[11]Cf. Kekulé's Ann. (1857), 104, 132n.
[12]Originally suggested by Williamson. (A.)
 Back to the list of selected historical papers.
Back to the list of selected historical papers.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.
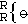 . In organic chemistry there should be oxides corresponding to ethylene oxide, just as there are representatives of ethyl oxide and of others that correspond to glycerol oxide, and if potassium hydrate, for example, can be compared to alcohol, then other hydrates should be compared with glycol and glycerine. It is understandable that these considerations are such as to prompt and justify some changes in Gerhardt's notation and in the atomic weights that he attributed to certain metals.
. In organic chemistry there should be oxides corresponding to ethylene oxide, just as there are representatives of ethyl oxide and of others that correspond to glycerol oxide, and if potassium hydrate, for example, can be compared to alcohol, then other hydrates should be compared with glycol and glycerine. It is understandable that these considerations are such as to prompt and justify some changes in Gerhardt's notation and in the atomic weights that he attributed to certain metals. Back to the list of selected historical papers.
Back to the list of selected historical papers.