Franz Hofmeister (1850-1922)
On the structure and grouping of the protein bodies
Ergebnisse der Physiologie 1, 759 (1902) [as translated and excerpted in Mikulás Teich, A Documentary History of Biochemistry, 1770-1940 (Rutherford, NJ: Fairleigh Dickinson University Press, 1992)]
...
Of the decomposition products of typical proteins which, with as simple a structure as possible, still contain more than one nucleus, only arginine and leucine imide have a constitution exactly known to us. The manner in which the carbon nuclei are united is seen in the following formulae:
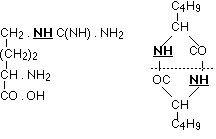
In both cases it is an imino group which joins the two carbon nuclei together, on the one hand the guanidine residue with ornithine, on the other two leucine complexes. The similarity of these ways of binding comes out even more clearly if only the carbons directly taking part are considered. Then we have the complexes:
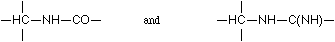
In fact, as is shown below, a corresponding scheme for binding applies particularly to proteins.
... the protein molecule is mainly buildup from amino acids, amongst which again the monoamino acids preponderate. A linking of the amino acids with one another by binding of carbon -C-C- would allow the giant protein molecule to appear as a single enormous branched carbon chain. Its decomposability, but particularly fermentative breakdown to definite larger and smaller complexes, would be most difficult to comprehend. The breakdown of longer into shorter carbon chains by animal proteolytic ferments, even with the very active trypsin, has not been observed.[1]
Likewise an ether or ester type of linkage =C-C-C=, such as O. Nasse[2] was inclined to assume on grounds of the analogy of fermentative protein breakdown with the diastatic and fat-splitting action of some enzymes also can scarcely be considered, if one realises that the alcohol (OH) groups necessary for such a linkage have not been demonstrated in protein (apart from the hydroxyl of tyrosine), and thus are either lacking or may be only very sparsely present. Also an ester type of interaction of the carboxyl groups contained in the amino acid complex would (in contradiction to the observed facts) undoubtedly give a basic character for the whole molecule since, as Curtius[3] has first shown, in the amino acids after esterification, the acid-binding character of the NH2 groups stand out strongly. On this ground also linkage in the manner of an acid anhydride is not to be considered.
Binary linking being presumed, the assumption of a binding in the manner 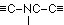 appears much more probable, offering from the outset series of possibilities. If one neglects the sulphur, only sparingly present in protein, one may think of the following cases:
appears much more probable, offering from the outset series of possibilities. If one neglects the sulphur, only sparingly present in protein, one may think of the following cases:
| I | II | III
|
| -CH2-NH-CH2 | -CH2-NH-CO- | -CH2-NH-C(NH)- |
With ternary binding still other assumptions could be made, for example
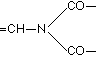 and the like. For the moment these need not be considered, as being unsuitable at the present time for fruitful discussion.
and the like. For the moment these need not be considered, as being unsuitable at the present time for fruitful discussion.
Against the more widespread distribution of a linkage according to Scheme I (which does in fact appear in individual nuclei, e.g. in the pyrrolidine nucleus) there is the circumstance that in this case (e.g. by analogous binding of two monoamino acids) the numerous carboxyl groups remaining free in the amino acid complex must give a strongly acid character to the whole molecule, which does not correspond to the facts.
Scheme III corresponds to the binding of the guanidine residue in arginine. As however the guanidine residue appears to occur in protein only in this single complex, the manner of binding in the building up of the protein molecule may be considered as of significance limited to the presence of arginine.
On the other hand there are numerous facts which speak for the very general distribution of a linkage according to Scheme II: -CH2-NH-CO- (or -NH-CH2-CO-NH-), which indeed is realized in leucine imide.
- As we have just shown, only a small part of the total nitrogen of protein bodies is present in a form readily split off, which could therefore correspond to the binding method -CO-NH2. By far the greatest part of the nitrogen, up to over 90% (judging from the end-products), must be present in a form yielding on hydrolysis a small part as imine (from arginine), by far the greater part in the amino form. That indeed only a small amount of NH2 groups are preformed in protein is shown in their behaviour with nitrous acid. According to O. Löw[4] and H. Schiff[5] only a relatively small part of the nitrogen is split off by this acid. As C. Paal[6] confirmed on the similarly constituted glutin peptone, substances behaving like nitrosamines are formed. Thus it is to be assumed that in the original protein molecule the NH2 groups of the end products are preformed by NH groups.
- The biuret reaction, which is considered specially characteristic for protein bodies, occurs according to Schiff,[7] with those substances which contain two CO-NH2- complexes, or instead of these CS.NH2- or C(NH).NH2-; and in certain circumstances also with -CH2.NH2- complexes bound through their carbon atoms, or through intervention of a carbon atom (in a CH2-, CH(OH)-, CO-group), or a nitrogen (in an NH- group).
A formulation corresponding to this method of binding of the protein-contained a-amino acids, as well as to the arrangement favourable to the biuret reaction is conceivable in the simplest manner in the following way, where in the interest of simplicity the coupling of leucine and glutamic acid may serve as an example:
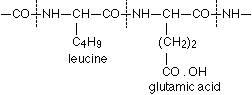
It is plain without further discussion that this manner of coupling with formation of the group 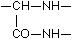 can repeat itself in the protein as often as an a-amino acid unites itself in the given manner with a second. Now are the glycine amide
can repeat itself in the protein as often as an a-amino acid unites itself in the given manner with a second. Now are the glycine amide
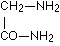 as well as sarcosine amide
as well as sarcosine amide 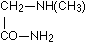 according to Schiff, aspartic acid diamide
according to Schiff, aspartic acid diamide
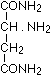 according to E. Fischer, bodies which give an intense biuret reaction. Thus they are relevant to the assumption that in the intact protein molecule (as well if not better in its near derivatives, the albumoses and peptones) the condition for the appearance of the biuret reaction is given by the presence of these
according to E. Fischer, bodies which give an intense biuret reaction. Thus they are relevant to the assumption that in the intact protein molecule (as well if not better in its near derivatives, the albumoses and peptones) the condition for the appearance of the biuret reaction is given by the presence of these 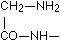 groups.
groups.
That nitrous acid without splitting the protein molecule causes this reaction to vanish is then immediately understandable through its known action on the NH2- and NH- groups; there is a similar explanation for the effect of alkali, which, easily saponifying the CONH2-groups, strikingly diminishes this reaction.[8].
- Recent experience on the condensation of amino acids leads to similar conceptions about the linkage of carbon nuclei[9]. Schaal in 1871 by boiling aspartic chloride at 200° in a stream of carbonic acid obtained condensation products--Schiff later named them polyaspartic acids--which as Grimaux[10] found, gave the biuret reaction. Through fusion of asparagine with urea the latter first obtained artificial colloids which, besides other protein reactions also gave the biuret reaction splendidly. Starting from glycinethylester Curtius then obtained a series of condensation products amongst which one of basic nature gave the biuret reaction. However also through addition of glycocoll to hippuric acid Curtius[11] arrived at complicated bodies, of which one presented the same reaction. On the way in which these condensations result from the aminoacetic acid and other amino acids we are now sufficiently instructed by Emil Fischer. The simplest compounds of this kind, which of course only partly give the biuret reaction, e.g. the glycylglycine, are built according to the type:[12]
NH2.(CH2.CO.NH)nCH2.CO.OH
Also the Curtius base has shown itself after closer research by M. Schwarzschild[13] to be an ester of a compound built just according to this type, NH2.(CH2CONH)6CH2.CO2.C2H5. All these facts teach that the amino acids are capable to an extraordinary degree of condensing in vitro in the specified manner to the complexes of high molecular weight.
- The coupling through formation of the -CH2-NH-CO- group, which in these compounds plays so great a role--and the -CH2-NH-C(NH)- group probably equivalent in this connection--is also very often met in vivo. One of the most familiar syntheses in the animal body, that of hippuric acid, C6H5.CO.NH.CH2COOH and its homologues follows this course; as well as the synthesis of the coupled bile acids and the vital formation of the uramino acids through the attachment of the -CO.NH2-residue to the NH2-group of an amino acid and the attachment of acetyl to the same place. This is, however, of significance inasmuch as it opens up an understanding as to how the organism proceeds with the building up of adequate protein substances by means of the protein-breakdown material flowing out of the intestine [into the blood]. On the other hand, hippuric acid like the proteins is accessible to the hydrolytic action of acid and alkali as well as the ferments (histozyme and bacterial enzymes). If in the former case hydrolytic splitting of the -CO-NH.CH2- linkage takes place, so it becomes probable that also with the protein an analogous manner of binding occurs. Besides Schwarzschild[14] lately succeeded in showing that a synthetic amino acid derivative built up in this way (the Curtius base mentioned above ) loses the biuret reaction on treatment with pure trypsin; this is the more remarkable as trypsin is not capable of splitting off ammonia from acid amides, e.g. acetamide or asparagine.
The type of condensation described here through formation of -CO-NH.CH= groups may thus explain both the building up of protein substances in the organism, as well as their breakdown in the intestinal tract and in the tissues.
On the basis of these given facts one may therefore consider the proteins as for the most part arising by condensation of a-amino acids, whereby the linkage through the group -CO-NH-CH= has to be regarded as the one regularly recurring.
[1]So far as such fermentative breakdown is known at all, it concerns however the otherwise well reported specific case of oxidation, where a terminal carboxyl splits off in the form of CO2. [original note]
[2]Nasse, O., Über die Wirkung der Fermente. Rostocker Zeitung, 15 Dec. 1894. [original note]
[3]Curtius, Th. and Göbel, Fr., Über Glykokolläther. Journ. f. prakt. Chemie, 37, 150. [original note]
[4]Loew, O., Über das Eiweiss und die Oxydation desselben. Journ. f. prakt. Chemie, 31, 129. [original note]
[5]Schiff, H., Über Desamidoalbumin, Ber. d. deutsch. chem. Ges. 29, 1354. [original note]
[6]Paal, Über die Peptonsalze des Glutins, Ber. d. deutsch. chem. Ges, 25, 1235. [original note]
[7]Schiff, H., Biuretreaktionen. Ber. d. deutsch. chem. Ges. 29, 298; Über Polyaspartsäuren. Ibid., 30, III. 2449 and Liebigs Annalen, 299, 236 and 319, 300. [original note]
[8]In this way also is explained the apparent contradiction that the biuret reaction of the protein is already abolished by nitrous acid with only a small evolution of nitrogen, although the appearance of split products giving an exquisite biuret reaction indicates wide occurrence of the group concerned in the reaction. In fact, it appears according to Schiff that the presence of NH2 end-groups is necessary: it is easy to see that by hydrolysis of the CONH.CH.groups NH2-groups must always be formed again from the protein. [original note]
[9]Schaal, Ed., Über einige Derivate der Aspartsäure. Liebigs Annal. 157, 24. [original note]
[10]Grimaux, Ed., Sur des colloides azotes. Bull. soc. chim., 1882, 38, 64. [original note]
[11]Curtius, Th., Über einige neue der Hippursäure analog konstituierte synthetisch dargestellte Amidosäuren. Journ. f. prakt. Chem. 26, 145. [original note]
[12]The compound C2H5.CO2.HN.CH2.CO.NH.CH2.CO.NH2 gives the biuret reaction as does an analogous compound, which contains a leucine residue. [original note]
[13]From still unpublished experiments. [original note]
[14]A communication to appear shortly will go further into this. We will only mention that a specially purified trypsin was used, completely free from substances giving the biuret reaction as well as from steapsin. [original note]
 Back to the list of selected historical papers.
Back to the list of selected historical papers.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.
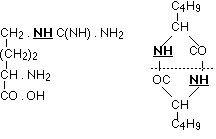
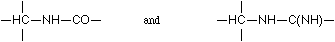
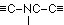 appears much more probable, offering from the outset series of possibilities. If one neglects the sulphur, only sparingly present in protein, one may think of the following cases:
appears much more probable, offering from the outset series of possibilities. If one neglects the sulphur, only sparingly present in protein, one may think of the following cases:
 and the like. For the moment these need not be considered, as being unsuitable at the present time for fruitful discussion.
and the like. For the moment these need not be considered, as being unsuitable at the present time for fruitful discussion.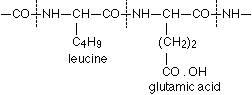
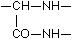 can repeat itself in the protein as often as an a-amino acid unites itself in the given manner with a second. Now are the glycine amide
can repeat itself in the protein as often as an a-amino acid unites itself in the given manner with a second. Now are the glycine amide
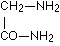 as well as sarcosine amide
as well as sarcosine amide 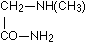 according to Schiff, aspartic acid diamide
according to Schiff, aspartic acid diamide
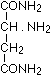 according to E. Fischer, bodies which give an intense biuret reaction. Thus they are relevant to the assumption that in the intact protein molecule (as well if not better in its near derivatives, the albumoses and peptones) the condition for the appearance of the biuret reaction is given by the presence of these
according to E. Fischer, bodies which give an intense biuret reaction. Thus they are relevant to the assumption that in the intact protein molecule (as well if not better in its near derivatives, the albumoses and peptones) the condition for the appearance of the biuret reaction is given by the presence of these 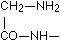 groups.
groups. Back to the list of selected historical papers.
Back to the list of selected historical papers.