Paul J. Crutzen (1933-)
"The influence of nitrogen oxides on the atmospheric ozone content", Quarterly Journal of the Royal Meteorological Society 96, 320-325 (1970).
Copyright ©1970. Posted with the permission of the author, the Royal Meteorological Society, and the Journal.
P. J. Crutzen*, Clarendon Laboratory, Oxford University
(Manuscript received 5 November 1969, communicated by Dr. C. D. Walshaw)
SUMMARY
The probable importance of NO and NO2 in controlling the ozone concentrations and production rates in the stratosphere is pointed out. Observations on and determinations of nitric acid concentrations in the stratosphere by Murcray, Kyle, Murcay and Williams (1968) and Rhine, Tubbs and Dudley Williams (1969) support the high NO and NO2 concentrations indicated by Bates and Hays (1967).
Some processes which may lead to production of nitric acid are discussed.
The importance of O(1S), possibly produced in the ozone photolysis below 2340 Å, on the ozone photochemistry is mentioned.
1. INTRODUCTION
It has long been assumed that the main reaction which balanced the production of odd oxygen particles by photodissociation of molecular oxygen was that between atomic oxygen and ozone. In recent years it has become clear, however, that this reaction is not sufficiently fast (Schiff 1969). In a search for other destruction mechanisms reactions between OH, HO2, and O3 have been proposed (Hunt 1966; Hampson 1965). It has, however, been indicated in a previous study (Crutzen 1969) that this hypothesis does not succeed in explaining the ozone observations between 30 and 35 km.
Bates and Hays (1967) have indicated that N2O, possibly produced by microbiological action in the soil and diffusing upwards through the troposphere, may partly be converted to 'odd nitrogen' (NO and NO2) by a photodissociation process in the stratosphere. As will be shown in the paper, on this hypothesis the NO and NO2 concentrations have a direct controlling effect on the ozone distributions in a large part of the stratosphere, and consequently on the atmospheric ozone production rates.
Recently measured nitric acid concentrations in the stratosphere (Murcray et al. 1968 and Rhine et al. 1969) tend to support the suggestions by Bates and Hays (1967).
2. REACTION SCHEME
| (1) | NO + O3 --> NO2 + O2 | | k1
|
| (2) | NO2 + hν --> NO + O | λ < 3975 Å | j2
|
| (3) | NO2 + O --> NO + O2 | | k3
|
| (4) | O2 + hν --> 2 O | λ < 2425 Å | j4
|
| (5) | O + O2 + M --> O3 + M | | k5
|
| (6) | O + O3 --> 2 O2 | | k6
|
| (7) | O3 + hν --> O + O2 | 3080 Å < λ < 11400 Å | j7
|
| (8) | O3 + hν --> O(1D) + O2 | λ < 3080 Å | j8
|
| (9) | O(1D) + M --> O + M | | k9
|
| (10) | O(1D) + H2O --> 2 OH | | k10
|
| (11) | OH + O -> H + O2 | | k11
|
| (12) | H + O2 + M --> HO2 + M | | k12
|
| (13) | HO2 + O --> OH + O2 | | k13
|
| (14) | OH + O3 --> HO2 + O2 | | k14
|
| (15) | OH + OH --> H2O + O | | k15
|
| (16) | OH + HO2 --> H2O + O2 | | k16
|
| (17) | HO2 + HO2 --> H2O2 + O2 | | k17
|
| (18) | H2O2 + OH --> HO2 + H2O | | k18
|
| (19) | H2O2 + hν --> 2 OH | λ < 5650 Å | j19
|
The following rate coefficients (expressed in the centimeter molecule second system) have been applied:
| k1 = 1.7x10-12 exp(-1,310/T) | , | Schofield (1967) |
| j2 = 5x10-3 | , | Nicolet (1965) |
| k3 = 3.2x10-11 exp(-530/T) | , | Schofield (1967) |
| k5 = 8x10-35 exp(445/T) | , | Benson-Axworthy (1965) |
| k6 = 5.6x10-11 exp(-2,850/T) | , | Benson-Axworthy (1965) |
| k9 = 4x10-11 | , | Zipf (1969) |
| k10 = 2x10-10 (assumed) |
|
| k11 = 5x10-11 | , | Kaufman (1969) |
| k12 = 4x10-32 | , | Schofield (1967) |
| k13 = 2x10-11 | , | according to Kaufman (1969): k13 ≥ 10-11
|
| k14 = 10-13 | , | according to Kaufman (1969): k14 .less than. 5x10-13
|
| k15 = 1.4x10-11 exp(-500/T) | , | Schofield (1967) |
| k16 = 10-11 | , | according to Kaufman (1969): k16 ≥ 10-11
|
| k17 = 3x10-12 | , | Schofield (1967) |
| k18 = 1.6x10-11 exp(-900/T) | , | Baulch, Drysdale and Lloyd (1969). |
The water vapour mixing ratio assumed in this study was 5x10-6. Concentrations of nitrogen oxides are taken from Bates and Hays (1967) and are given in the Table. Absorption cross-sections for ozone and molecular oxygen are from Vigroux (1953) and Brewer-Wilson (1965). Solar flux data have been taken from Brewer-Wilson (1965) and Johnson (1954).
It may be noticed that the very speculative reaction between HO2 and O3 is not used in the above scheme.
3. THE PHOTOCHEMICAL EQUATIONS
As shown by Nicolet (1965), NO and NO2 are in mutual equilibrium through the reactions (1) to (3) and therefore
| (20) | 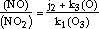 |
where quantities within parentheses denote concentrations. This is even more exact for O2 and O3 through reactions (5), (7) and (8).
| (21) | 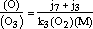 |
Making use of Eq. (20) we write for the time rate of change of odd oxygen and therefore ozone below 50 km
| (22) | d(O3)/dt = P - (D1+D2+D3) |
| where |
|
| (23a) | P = 2j4(O2) |
| (23b) | D1 = 2k3(O)(NO2) |
| (23c) | D2 = (k11(O)+k14(O3))(OH) + k13(O)(HO2) |
| (23d) | D3 = 2k6(O)(O3) |
4. RESULTS
The daily production and destruction for odd oxygen below 50 km have been estimated for conditions at the Equator. The observed ozone distribution was taken from Dütsch (1964). The results of the computations are shown in Table 1.
It can be seen that the destruction of odd oxygen by the nitrogen oxides is of the same order of magnitude as the production by photodissociation of molecular oxygen. Reductions of odd oxygen by odd nitrogen is, according to these estimates, dominant between approximately 25 and 40 km. There are, however, indications in the Table that reduction by OH and HO2 begins to be of larger importance around the stratopause.
5. NITRIC ACID
The detection of nitric acid (HNO3) in the ozonosphere by Murcray, Kyle, Murcray and Williams (1967) and the deduced number mixing ratios (approx. 3x10-9) provide us with very valuable information.
Nitric acid may be formed by the pair of reactions (see Leighton 1961; Nicolet 1965):
| (24) | OH + NO2 + M --> HNO3 + M |
| (25) | HO2 + NO + M --> HNO3 + M |
| which are followed by |
|
| (26) | HNO3 + hν --> OH + NO2 |
These reactions occur mainly during daytime, because OH and NO are removed at night by reactions (1) and (14). Reaction rates k24 and k25 are not known, but to estimate expected maximum concentrations the value 10-31 has been assigned to them. The absorption of nitric acid has been measured by Dalmon (1943) between 2300 and 3100 Å. His data indicate a rise at shorter wavelengths, which is difficult to estimate. Leighton (1961) lists the value of 5x10-6 for calculations in polluted air at ground level. The value applied in this study, 10-5, is uncertain and may be too low. The derived maximum HNO3 mixing ratios are listed in the Table and are of the same order of magnitude as the concentrations observed by Rhine, Tubbs and Dudley Williams (1969). This is a clear indication that the nitrogen oxide concentrations are in fact of the order as those given by Bates and Hays (1967). It is interesting to note the possibility that in the lower stratosphere a large portion of the nitrogen oxides may appear as nitric acid. The actual NO and NO2 concentrations in the stratosphere may be larger than those used here without leading to a surplus of the destruction over the production of odd oxygen. In the first place there is still uncertainty about the solar flux around 2100 Å, where Detwiler et al. (1961) give data which are almost three times larger than those used here. Furthermore the exact value of the rate coefficient k5 is not well known and consequently the atomic oxygen concentrations may be lower than those used in this study.
It should also be pointed out that the water vapour mixing ratios may be different from those assumed here.
It can, of course, not be excluded that additional processes must be considered in the HNO3 formation. A possibility for more OH and HO2 are the processes
| (27) | O3 + hν --> O(1S) + O2(3Σg-), | λ ≤ 2340 Å |
| followed by | |
|
| (28) | O(1S) + H2O --> 2 OH |
|
| k28 = 3x10-10 | Zipf (1969). |
Reaction (27) is spin forbidden, but a quantum yield as low as 10-2 may be enough to make it of interest. The reason for this is that O(1S) is much less rapidly deactivated than O(1D):
| (29) | O(1S) --> O2 --> O + O2 |
|
| (30) | O(1S) + N2 --> O + N2 |
|
| k29 ≥ 5x10-13, | Zipf (1969) |
| k30 ≥ 1.3x10-15, | Zipf (1969). |
It cannot be dismissed that more OH will be produced by reaction (28) than by reaction (10) in parts of the stratosphere, in which case it may even be of direct importance for the ozone distribution (reactions (11) to (14)).
6. CONCLUSIONS
There is a distinct possibility that nitrogen oxides are of great importance in ozone photochemistry. In the first place we urgently need observations on their concentrations in the stratosphere. Investigations about the photodissociation products of N2O and its origin (see Bates and Hays 1967) should be continued and extended in order to establish if N2O is an important source for odd nitrogen in the upper atmosphere. If, however, most of the stratospheric NO and NO2 is produced at very high levels by other processes some solar cycle influence on the ozone layer will be possible.
Another question which should be investigated is what products are formed in the photolysis of ozone below 2400 Å, in particular whether O(1S) is formed.
The concentrations of nitric acid reported by Rhine et al. (1969) can be explained by considering reactions between OH, HO2, NO and NO2 (reactions 24 and 25), although other possibilities cannot be dismissed. Too little is known about reactions occurring in a nitrogen-oxygen-hydrogen atmosphere.
ACKNOWLEDGMENT
The author wishes to express his gratitude to the European Space Research Organization for a post-doctoral fellowship.
REFERENCES
- Bates, D. R., Hays, P. B., 1967, 'Atmospheric nitrous oxide,' Planet. Space Sci., 15, pp. 189-197.
- Baulch, D. L., Drysdale, D. D. and Lloyd, A. C., 1969, 'High temperature reaction rate data', Report No. 3, Dept. of Physical Chemistry, The University, Leeds.
- Benson, S. W. and Axworthy, A. E., 1965, 'Reconsiderations of the rate constants from the thermal decomposition o ozone,' J. Chem. Phys., 42, pp. 2,614-2,615.
- Brewer, A. W. and Wilson, A. W., 1965, 'Measurements of solar ultraviolet radiation in the stratosphere,' Quart. J. R. Met. Soc., 91, pp. 452-461.
- Crutzen, P. J., 1969, 'Determination of parameters appearing in the "dry" and "wet" photochemical theories for ozone in the stratosphere,' Tellus, 21, pp. 368-388.
- Dalmon, M. R., 1943, 'Recherches sur l'acide nitrique et ses solutions per les spectres d'absorption dans l'ultraviolet,' Thèses présentées à la faculté des sciences de l'université de Paris.
- Detwiler, C. R., Garret, D. L., Pucell, J. D. and Tousey, R., 1961, 'The intensity distrubution in the ultraviolet solar spectrum,' Ann. de Geophys., 17, pp. 263- 272.
- Dütsch, H. U., 1964, 'Uniform evaluation of Umkehr observations from the world network,' Part III, N.C.A.R., Boulder, Colorado.
- Hampson, J. 1965, 'Chemiluminescent emission observed in the stratosphere and mesosphere,' Les problèmes météorologiques de la stratosphère et de la mésosphère, Presses Universitaires de France, pp. 393-440.
- Hunt, B. G., 1966, 'Photochemistry of ozone in a moist atmosphere,' J. Geophys. Res., 71, pp. 1,385- 1,398.
- Johnson, F. S., 1954, 'The solar constant,' J. Met., 11, pp. 431-439.
- Kaufman, F., 1969, 'Neutral reactions involving hydrogen and other minor constituents,' Can. J. Chem., 47, pp. 1,917-1,927.
- Leighton, P. A., 1961, Photochemistry of air pollution, Academic Press, New York.
- Murcray, D. G., Kyle, T. G., Murcray, F. H. and Williams, W. J., 1968, 'Nitric acid and nitric oxide in the lower stratosphere,' Nature, 218, pp. 78-79.
- Nicolet, M., 1965, 'Nitrogen oxides in the chemosphere,' J. Geophys. Res., 70, pp. 679-689.
- Rhine, P. E., Tubbs, L. D. and Dudley Williams, 1969, 'Nitric acid vapour above 19 km in the earth's atmosphere,' App. Optics, 8, pp. 1,500-1,501.
- Schiff, H. I., 1969, 'Neutral reactions involving oxygen and nitrogen,' Can. J. Chem., 47, pp. 1,903- 1,916.
- Schofield, K., 1967, 'An evaluation of kinetic rate data for reactions of neutrals of atmospheric interest,' Planet Space Sci., 15, pp. 643-670.
- Vigroux, E., 1953, 'Contibution à l'étude expérimentale de l'absorption de l'ozone,' Ann. de Phys., 8, pp. 709-763.
- Zipf, E. C., 1969, 'The collisional deactivation of metastable atoms and molecules in the upper atmosphere,' Can. J. Chem., 47, pp. 1,863-1,870.
*ESRO post-doctoral fellow on leave from the University of Stockholm, Sweden.
Table 1. Number concentrations (cm-3) and volume mixing ratios of some molecules and estimated production (P) and destruction rates (D1, D2 and D3) over one day.
(8 (10)=8x1010)
[Note: I have divided Table 1 into two parts for ease of "typesetting". CJG]
Concentrations at noon per cm3
| Altitude in km | O3 | O | NO | NO2 | OH | HO2
|
|---|
| 50 | 8 (10) | 6 (9) | 2.6 (9) | 9 (7) | 2 (7) | 5 (7) |
| 35 | 1.2 (12) | 2.8 (8) | 5.8 (9) | 7.5 (9) | 4.7 (6) | 1 (8) |
| 30 | 2.6 (12) | 9 (7) | 4.2 (9) | 1.1 (10) | 2.2 (6) | 2 (8) |
| 25 | 4.4 (12) | 2.7 (7) | 2.9 (9) | 1.1 (10) | 7.4 (5) | 1.9 (8) |
| 20 | 3.3 (12) | 4.9 (6) | 2 (9) | 5 (9) | 3.2 (5) | 1.3 (8) |
| 15 | 1 (12) | 3.7 (5) | 3 (9) | 2.4 (9) | 2.8 (5) | 6.9 (7) |
| Altitude in km | Volume mixing ratio NO, NO2, HNO3 | Volume mixing ratio HNO3 | ∫ Pdt | ∫ D1dt | ∫ D2dt | ∫ D3dt |
|---|
| 50 | 1 (-7) | 1.6 (-9) | 4 (11) | 2 (11) | 4.8 (11) | 5 (10) |
| 35 | 7 (-8) | 6.3 (-9) | 2.7 (11) | 4.7 (11) | 4.5 (10) | 8 (9) |
| 30 | 4 (-8) | 8.7 (-9) | 1.5 (11) | 2.4 (11) | 4 (10) | 1.6 (10) |
| 25 | 2.5 (-8) | 7 (-9) | 7.2 (10) | 7.2 (11) | 1.8 (10) | 2.4 (9) |
| 20 | 1 (-8) | 3.7 (-9) | 1.7 (10) | 4 (9) | 4 (9) | 3 (8) |
| 15 | 3 (-9) | 2 (-9) | 6 (8) | 2 (8) | 1 (9) | 4.8 (6) |
 Back to the list of selected historical papers.
Back to the list of selected historical papers.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.
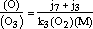
 Back to the list of selected historical papers.
Back to the list of selected historical papers. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.