Mariotte's law
Edme Mariotte was one of the first members of the French Academy of Sciences. He carried out research in many areas of the field now known as physics. This exercise involves his observations on the relationship between the pressure and volume of gases.
Mariotte described a set of observations summarized below and illustrated by the accompanying figure.
- The height of a column of mercury supported by the atmosphere was found to be 28 pouces (a unit of length a little longer than an inch). So we can say that the pressure of the atmosphere, in Mariotte's units, was 28 pc Hg.
- Mariotte then poured mercury into another tube with a long open end and a short closed end. He poured just enough mercury into the tube to separate the air in the closed end from the open end. The height of the column of air in the closed end was 12 pouces, so the volume of the air was 12 pouces times the cross sectional area of the tube. Since the cross section of the tube is constant, the volume of air in the closed end of the tube is proportional to its height, and we can say that the "volume" is 12 pc. The pressure of the air in the closed end is the same as the pressure of the atmosphere.
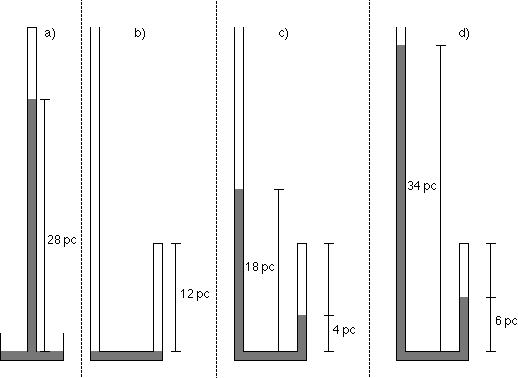
- Mariotte poured some more mercury into the tube, stopping when the column of mercury in the closed end of the tube was 4 pc above the level of the bend. He noticed that the mercury in the open end of the tube was 18 pc above the bend, or 14 pc higher than the column in the closed end. The pressure of the air in the closed end is the pressure of the atmosphere plus that of the "excess" mercury pushing on it. The "excess" pressure is the difference in the heights of the columns of mercury in the open and closed ends of the tube, namely 14 pc Hg.
- Mariotte poured more mercury into the tube, stopping when the column of mercury in the closed end of the tube was 6 pc above the bend. He noticed that the mercury in the open end of the tube was 34 pc above the bend.
- Mariotte poured still more mercury into the tube, stopping when the column of mercury in the closed end of the tube was 9 pc above the bend.
1) Make a table of the pressure and "volume" of the air trapped in the closed end of the tube. Use Mariotte's observations to fill in the values of "volume" (height of the air column) for parts b, c, d, and e. Use his observations to fill in the values of the pressure (atmospheric pressure plus excess height of mercury) for parts b, c, and d.
2) Verify that the pressure and "volume" obey Boyle's law for parts b, c, and d.
3) Use Boyle's law to compute the pressure for part e (in pc Hg).
4) Assuming that the open end of the tube is tall enough, how high (in pc) is the column of mercury in the open end (how much above the bend)?
Reference
Edme Mariotte on the nature of air (1676, 1679) as excerpted in William Francis Magie, A Source Book in Physics (New York: McGraw-Hill, 1935), pp. 88-92.
Copyright 2003 by Carmen Giunta. Permission is granted to reproduce for non-commercial educational purposes.
| Back to the top of the Classic Chemistry site |

