Soddy received the Nobel Prize for chemistry in 1921 for his work on isotopes. In his lecture at the award ceremony, he said of isotopes, "Put colloquially, their atoms have identical outsides but different insides." [Soddy 1921] Atoms of the same element which are not identical in every particular represents a significant deviation from Dalton's concept of the atom (chapter 7); however, the idea had surfaced from time to time before research on radioactive elements made their presence manifest [Crookes 1886, Ramsay 1897]. The arduous and futile work[1] of attempting to separate by chemical means materials which clearly had different radioactive properties led to example after example of the phenomenon which was eventually recognized as isotopy. The excerpt reproduced below describes isotopes and the displacement law.
In the last section of the 1910 Report, "Chemical Relationships of the Radio-elements,"[3] the existence of groups of radio-elements possessing identical chemical properties was shown to foreshadow "some embracing generalisation which will throw light, not only on radioactive processes, but on the elements in general and the Periodic Law."[4] In 1911 the first step in this direction was made, when it was recognised that the expulsion of the α-particle causes the radio-element to change its position in the periodic table, not into the next family, but into the next but one in the direction of diminishing group number and diminishing atomic mass.[5] Last year doubtful points in the sequence of changes[6], consequent upon the branching of the disintegration series at the C-members[7], and on the existence, in uranium, of two chemically identical elements, uranium-I and -II, were cleared up, and the important step made that the B- and C-members of the three series exhibit identical electrochemical behaviour. [8], [9] In the meantime, a systematic study of the chemical nature of those disintegration products not hitherto thoroughly studied from a chemical point of view had resulted in a remarkable extension of the feature which dominates the chemistry of the radio-elements.[10] Radioactinium was shown to be chemically identical with thorium; mesothorium-II with actinium; the three B-members and radium-D with lead; the three C-members and radium-E with bismuth; thorium-D and actinium-D with thallium[11]; and radium-A with polonium. Thus, not a single one of the radio-elements, known at the commencement of the year, has a peculiar chemical nature unshared by others. All are chemically indistinguishable from one or other of the elements occupying the last twelve places of the periodic table, from thallium to uranium.[12] With the sequence of changes fully elucidated and the chemical character of the majority of the radio-elements established, the α-ray rule was shown to hold generally, and, equally generally, a similar rule for the β-ray changes was found to apply. In the β-ray change, the element shifts its position in the periodic table in the opposite direction to that in the α-ray change, but into the next family, not into the next but one.[13] These two simple rules[14], consistently applied to the three disintegration series, constitute a sweeping generalisation connecting the chemical character of the radio-element, and the position it occupies in the periodic table with the kind of radioactive change in which it is produced. In addition to the purely chemical discoveries considered, an electrochemical examination of the radio-elements led independently to the same generalisation. It was found that the expulsion of the α-particle resulted in a product more electro-positive, and of a β-particle more electro-negative, than the parent.[15]
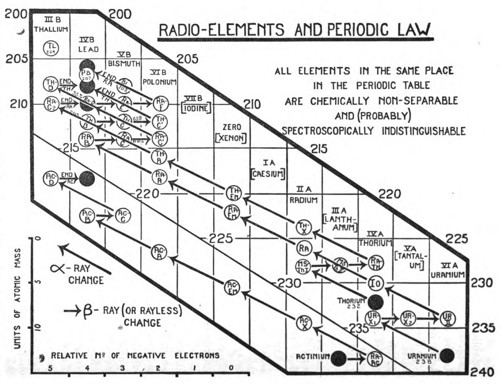
The generalisation is illustrated in the chart (Fig. 1).[16] This satisfactorily accounts for all the peculiar features that characterise the chemistry of the radio-elements. Whenever, by the expulsion of α- or β-rays, two or more elements come to occupy the same place in the periodic table, then, independently of all other considerations, such as the atomic weight, the disintegration series to which the element belongs, its radioactive character and the nature of the radioactive changes, in which it is produced, or by which it is transformed, these elements, occupying the same place, are non-separable from one another, and are, so far as is known, identical in chemical character. Each vertical row of the diagram consists of such a group of chemically identical elements. The ten occupied places contain over forty distinct elements, whereas if chemical analysis alone had been available for their separate recognition, only ten elements could have been distinguished.[17] The places at the end of the periodic table, and probably elsewhere in the table, thus represent, not single homogeneous elements as has hitherto been supposed, but groups of elements identical in chemical character. To express this newly discovered complexity of matter, the terms "isotopic elements" or "isotopes" have been coined.[18] Thus radiothorium, ionium, thorium, uranium-X1, and radioactinium are a group of isotopic elements, the calculated atomic masses of which vary from 228 to 234. They all occupy the same place in the periodic table, and are chemically indistinguishable. This material identity, however, extends far beyond the chemical properties in the narrow sense, and embraces probably nearly all the common physical properties also, so that the experimental means capable of distinguishing and separating isotopes are very limited.[19], [20] Thus, eleven years ago, the result that the radium and thorium emanation condense at practically the same temperature seemed very extraordinary. Now there is every reason to believe that isotopes will prove to be indistinguishable in volatility no less than in chemical properties. The question whether isotopes have the same spectrum, for example, was discussed for the case of ionium and thorium last year.[21], [22] Important new evidence can be urged both for and against the general view. The recent generalisation that the magnitude of the atomic weight enters exactly into the series relationships of spectra, and the expectation that has been advanced that, ultimately, it should be possible to determine atomic weights from these series relationships more accurately than by chemical analysis,[23] is obviously opposed to the possibility that elements of different atomic masses can have the same spectra.[24]
Neon and Metaneon.--On the other hand, what appears to be a case of isotopic elements outside the radioactive sequences has been discovered.[25] As has been remarked, very few material properties depend directly on atomic mass. Fractional diffusion of gases is almost the only property that can be expected to effect a partial separation of a group of isotopic elements into their constituents. Whilst, to detect the non-homogeneity if it exists, the new positive ray method of Sir J. J. Thomson[26] is again almost the only one available.[27] The examination of atmospheric neon by the method revealed the presence of atoms, in relatively small proportion, of mass 22, in addition to the known atom of mass 20. The relative proportion of the two kinds of atoms was unchanged after a prolonged fractionation of the gas by cold charcoal; but fractional diffusion showed that atmospheric neon is not homogeneous, and a partial separation, attested by a change of density, was effected by this means. No change in the spectrum corresponding with the change of density was observed, however, and the two elements appeared to be identical in all properties, except atomic weight.[28]
This accords with what has been found in the case of ionium and thorium, as regards the spectra, and in the case of the radium and thorium emanation, as regards the volatility, and indicates that isotopes will prove to be identical in these respects as they are in chemical character. At the same time, the discovery is a most dramatic extension of what has been found for the elements at one extreme of the periodic table, to the case of an element at the other extreme[29], and strengthens the view that the complexity of matter in general is greater than the periodic law alone reveals. Although the complexity is greater, the problem of atomic structure has been much simplified, because the generalisation gives a probable explanation of the absence of exact simple numerical relations among the atomic weights.[30]
...[31]
[2]Soddy wrote reviews of recent progress in radioactivity as part of the Chemical Society Annual Reports series every year or two from the first report in 1905 through the nineteen-teens. The first few pages of Soddy's lengthy 1913 review article are reproduced here.
A review article differs from primary reports of scientific research in that it summarizes research on a particular topic carried out and published by many investigators. It does not contain detailed descriptions of experiments; instead it contains brief summaries of results and copious references to the articles in which the details were published. Thus, a review article allows the reader to "catch up" with recent work in a particular field, and provides citations to information in greater depth.
[3]At the end of his 1910 review, Soddy described the concept of isotopes, although he did not use the name: radioactive elements with different atomic weights but identical chemical properties. That paper summarized good evidence for the existence of isotopes among radioactive elements. Already it extended the likelihood of isotopes to non-radioactive elements: "The recognition that elements of different atomic weight may possess identical chemical properties seems destined to have its most important application in the region of inactive elements, where the absence of a second radioactive nature ... makes it impossible for chemical identities to be individually detected." [Soddy 1910]
[4]Ann. Report, 1910, 285. [original note --CJG]
[5]F. Soddy, "Chemistry of the Radio-elements," 1911, p. 30. [original note --CJG]
[6]The "changes" are chemical transformations which accompany radioactive decay. Three such sequences were known, each beginning with a different naturally-occuring radioactive element (designated by black circles at the lower right of the figure). The starting material would emit an α particle or a β particle, and change into a different element, which in turn would also emit an a or β particle, changing into yet another substance. After several radioactive decays, a stable atom would be formed, stopping the sequence (at the black circles on the upper left of the figure). Investigators labeled the intermediate materials with a variety of prefixes, suffixes, and descriptors, unsure if they were new elements and generally unable to characterize them very well. For example, the actinium sequence can be written:
actiniumradioactinium
actinium-X
actinium emanation
actinium-A
actinium-B
actinium-C
actinium-D
lead .
[7]A series was said to branch at a substance which could emit either an α or a β particle. For example, thorium-C (in the thorium series) was about twice as likely to emit a β particle (leading to thorium-C') as an α particle (leading to thorium D). The uranium series had a branch point at radium-C (hence Soddy writes, "branching ... at the C-members"). Naturally, the presence of a branch complicates matters, for the sequence which began with one element may now follow two different paths.
[8]Ann. Report, 1912, 311, 321, 319. Compare also E. Marsden and R. H. Wilson, Phil. Mag., 1913, [vi], 26, 354; A., ii, 907; P. Beer and K. Fajans, Physikal. Zeitsch., 1913, 14, 947; A., ii, 907; A. B. Wood, Phil. Mag., 1913, [vi], 26, 586; A., ii, 908; K. Fajans, Physikal. Zeitsch., 14, 951; A., ii, 908; G. von Hevesy and L. von Putnoky, Physikal. Zeitsch., 1913, 14, 63; Phil. Mag., 1913, [vi], 25, 415; A., ii, 175. [original note. The references given to "A." are to abstracts in Britain's Journal of the Chemical Society, for 1913 if no year is given.]
[9]That is, thorium-C, radium-C, and actinium-C could not be distinguished or separated from each other on the basis of electrochemical reactions; nor could thorium-B, radium-B, and actinium-B. It is important to recall that radioactivity is not considered a chemical phenomenon, for it is not a reaction involving an association or dissociation of atoms. Rather radioactivity is a nuclear phenomenon, involving changes of the core or nucleus of an atom. The distinction is an important one, in defining isotopes, which are identical chemically but have different nuclei. Here Soddy presents two sets of isotopes, two sets of materials which have identical chemical behavior (not just similar as in members of the same family in the periodic table) but different nuclei.
[10]A. Fleck, Chem. News, 1912, 106, 128; 1913, 107, 95; T., 1913, 103, 381, 1052. [original note --CJG]
[11]Compare also W. Metzener, Ber., 1913, 46, 979; A., ii, 375. [original note --CJG]
[12]"The feature which dominates the chemistry of the radio-elements" is that they all come in several varieties with different nuclei; i.e., they all have isotopes.
[13]A. S. Russell, Chem. News, 1913, 107, 49; A., ii, 274; K. Fajans, Physikal. Zeitsch., 1913, 14, 131, 136; Ber., 1913, 46, 422; A., ii, 276, 277; Ber. Deut. physikal. Ges., 1913, 15, 240; A., ii, 493; Le Radium, 1913, 10, 171; A., ii, 660; F. Soddy, Chem. News, 1913, 107, 97; Jahrb. Radioaktiv. Elektronik, 1913, 10, 188; A., ii, 275.
[14]The combination of the two rules is known as the displacement law.
[15]Electropositive and electronegative are two extremes of a continuum chemists have found useful in describing elements. An element is said to be electronegative to the extent that it holds on to negative electrical charge (electrons); conversely an element is called electropositive to the extent that it tends to lose electrons. In any given row of the periodic table, elements nearer the left are more electropositive and elements nearer the right more electronegative.
[16]The figure is certainly "busy." It contains a great deal of information, but some of that information is difficult to see. The numbers (the vertical scale) refer to atomic mass. The boxes on the horizontal scale refer to places on the periodic table. Although these boxes are extended vertically, each column represents a single place, a single element, and not a column or group of the periodic table.
The chart depicts all three disintegration series, beginning with actinium, uranium, and thorium in the lower right and ending with various isotopes of lead in the upper left. Starting and ending points are denoted by black circles. The short arrows pointing right represent β decay; this transformation does not change the mass number of the decaying atom, hence the horizontal arrow. The longer arrows pointing left represent α decay; this transformation lowers the mass number of the decaying atom by 4, hence the upward angle.
The open circles carry nearly illegible abbreviations for the various radio-elements. Most are short for thorium, radium, actinium, or uranium followed by a letter. These names, however, are of little interest except historically. The modern names for these substances recognize that circles in the same row are the same element. They are refered to by their element names (or symbols) and the mass number. Thus the five circles in the thorium column are 228Th (formerly radiothorium), 230Th (ionium), 232Th, 234Th (uranium-X1), 238Th (radioactinium). View a table of isotopes involved in the decay series and their former names.
[17]Today we might say that there are over forty distinct nuclides (atoms distinguished by their nuclear characteristics), but we would not say forty different elements. The modern definition of element depends on chemical properties, so chemically identical substances are samples of the same element, even if they contain distinctly different nuclides. (Actually, an element is now defined in terms of atoms with the same atomic number, which is a property which correlates to chemical properties.) Similarly, we would not call a collection of nuclides with identical chemical properties a group of elements, but a group of isotopes.
[18]Soddy coined the term isotope from Greek roots which mean "same place," for isotopes occupy the same place in the periodic table. Dr. Margaret Todd, a friend of Soddy's in-laws, suggested the term. [James 1993]
[19]"Chemistry of the Radio-elements. Part II. The Radio-elements and the Periodic Law." By F. Soddy: Longmans, Green & Co., 1914. [original note --CJG]
[20]Separation of isotopes is indeed difficult. Some natural processes actually do result in slight separations of isotopes. For example, the ratio of the heavier to lighter isotopes of oxygen in atmospheric water vapor varies slightly with temperature, allowing researchers to make inferences about past climates based on the measurement of isotope ratios in segregated samples of air. Artificial methods for separating isotopes are based on properties which depend on the mass of the atom, such as the rate of diffusion in gases. (See chapter 15, note 18; Ramsay believed at first that he had separated isotopes of helium by diffusion.)
[21]Ann. Report, 1912, 321. [original note --CJG]
[22]Soddy concluded his treatment on this question by saying, "The simplest, if somewhat heterodox, view to take is ... that its spectrum [i.e., of ionium], as well as its whole chemical behaviour, is identical with that of thorium."
[23]W. M. Hicks, Phil. Trans., 1913, A, 213, 323; A., ii, 810. [original note --CJG]
[24]The appearance of atomic spectra, it turns out, has practically no dependence on the mass of the atom. Isotopes are, as suggested by the chart, indistinguishable by their atomic spectra.
[25]This topic was a matter of speculation for Soddy three years ago (note 3 above); now he reports on the first evidence on the matter. Note how the discovery of isotopes among radioactive elements prompts the question of whether there are isotopes among stable elements. Isotopes were first noticed among the radioactive elements, because their very different radioactivities made investigators aware that they were different despite their chemical identities. Once the phenomenon is discovered among one group of elements, it is natural to look for it elsewhere.
[26]Sir J. J. Thomson, Bakerian Lecture, Proc. Roy. Soc., 1913, 89, A, 1; A., ii, 820. [original note --CJG]
[27]Thomson's device subjected positively charged ions of gaseous atoms to a magnetic field which bent the path of the ions to different extents depending on their masses. As might be guessed, heavier ions were bent less than light ones. Thomson's assistant, Francis W. Aston went on to develop a much more powerful device called a mass spectrograph. With his new device, Aston set about systematically searching stable elements for the existence of isotopes. Aston was awarded the Nobel Prize in Chemistry in 1922, one year after Soddy.
[28]F. W. Ashton: Paper communicated to the British Association, Section A., Birmingham, 1913. [original note. "Ashton," by the way, is Aston.--CJG]
[29]The naturally radioactive elements are among the heaviest elements, while neon is among the lighter ones. Isotopy, Soddy notes, is found at both ends of the periodic table.
[30]Soddy suggests that the reason atomic weights present no simple numerical pattern is because of isotopes. Recall that since Prout (chapter 10), several researchers represented in this volume had been concerned with finding relationships among atomic weights. They sought patterns, but none of the proposed patterns turned out to be perfectly regular. The atomic weight of an element is actually an average quantity, dependent on the weights of the individual isotopes of an element and their relative abundance. Since the atomic weight of an element is a complicated property of the element rather than a fundamental one, the irregularities in this quantity are not so troubling.
[31]I have omitted the remainder of the review article. --CJG
 Back to the top of the table of contents of Elements and Atoms.
Back to the top of the table of contents of Elements and Atoms. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.