Frederick Soddy (1877-1956)
Radioactivity
from Chemical Society Annual Reports 10, 262-88 (1913)
In the last section of the 1910 Report, "Chemical Relationships of the Radio-elements," the existence of groups of radio-elements possessing identical chemical properties was shown to foreshadow "some embracing generalisation which will throw light, not only on radioactive processes, but on the elements in general and the Periodic Law."[1] In 1911 the first step in this direction was made, when it was recognised that the expulsion of the α-particle causes the radio-element to change its position in the periodic table, not into the next family, but into the next but one in the direction of diminishing group number and diminishing atomic mass.[2] Last year doubtful points in the sequence of changes, consequent upon the branching of the disintegration series at the C-members, and on the existence, in uranium, of two chemically identical elements, uranium-I and -II, were cleared up, and the important step made that the B- C-members of the three series exhibit identical electrochemical behaviour.[3] In the meantime, a systematic study of the chemical nature of those disintegration products not hitherto thoroughly studied from a chemical point of view had resulted in a remarkable extension of the feature which dominates the chemistry of the radio-elements.[4] Radioactinium was shown to be chemically identical with thorium; mesothorium-II with actinium; the three B-members and radium-D with lead; the three C-members and radium-E with bismuth; thorium-D and actinium-D with thallium[5]; and radium-A with polonium. Thus, not a single one of the radio-elements, known at the commencement of the year, has a peculiar chemical nature unshared by others. All are chemically indistinguishable from one or other of the elements occupying the last twelve places of the periodic table, from thallium to uranium. With the sequence of changes fully elucidated and the chemical character of the majority of the radio-elements established, the α-ray rule was shown to hold generally, and, equally generally, a similar rule for the β-ray changes was found to apply. In the β-ray change, the element shifts its position in the periodic table in the opposite direction to that in the α-ray change, but into the next family, not into the next but one.[6] These two simple rules, consistently applied to the three disintegration series, constitute a sweeping generalisation connecting the chemical character of the radio-element, and the position it occupies in the periodic table with the kind of radioactive change in which it is produced. In addition to the purely chemical discoveries considered, an electrochemical examination of the radio-elements led independently to the same generalisation. It was found that the expulsion of the α-particle resulted in a product more electro-positive, and of a β-particle more electro-negative, than the parent.
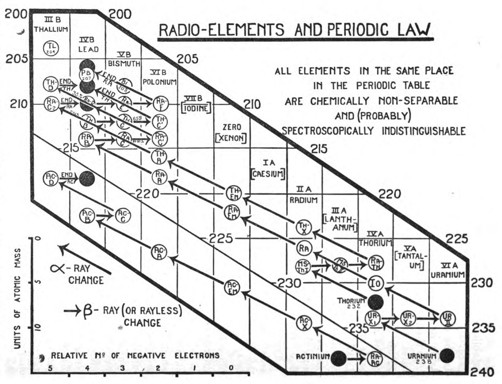
The generalisation is illustrated in the chart (Fig. 1). This satisfactorily accounts for all the peculiar features that characterise the chemistry of the radio-elements. Whenever, by the expulsion of α- or β-rays, two or more elements come to occupy the same place in the periodic table, then, independently of all other considerations, such as the atomic weight, the disintegration series to which the element belongs, its radioactive character and the nature of the radioactive changes, in which it is produced, or by which it is transformed, these elements, occupying the same place, are non-separable from one another, and are, so far as is known, identical in chemical character. Each vertical row of the diagram consists of such a group of chemically identical elements. The ten occupied places contain over forty distinct elements, whereas if chemical analysis alone had been available for their separate recognition, only ten elements could have been distinguished. The places at the end of the periodic table, and probably elsewhere in the table, thus represent, not single homogeneous elements as has hitherto been supposed, but groups of elements identical in chemical character. To express this newly discovered complexity of matter, the terms "isotopic elements" or "isotopes" have been coined. Thus radiothorium, ionium, thorium, uranium-X1, and radioactinium are a group of isotopic elements, the calculated atomic masses of which vary from 228 to 234. They all occupy the same place in the periodic table, and are chemically indistinguishable. This material identity, however, extends far beyond the chemical properties in the narrow sense, and embraces probably nearly all the common physical properties also, so that the experimental means capable of distinguishing and separating isotopes are very limited.[7] Thus, eleven years ago, the result that the radium and thorium emanation condense at practically the same temperature seemed very extraordinary. Now there is every reason to believe that isotopes will prove to be indistinguishable in volatility no less than in chemical properties. The question whether isotopes have the same spectrum, for example, was discussed for the case of ionium and thorium last year.[8] Important new evidence can be urged both for and against the general view. The recent generalisation that the magnitude of the atomic weight enters exactly into the series relationships of spectra, and the expectation that has been advanced that, ultimately, it should be possible to determine atomic weights from these series relationships more accurately than by chemical analysis,[9] is obviously opposed to the possibility that elements of different atomic masses can have the same spectra.
Neon and Metaneon.--On the other hand, what appears to be a case of isotopic elements outside the radioactive sequences has been discovered. As has been remarked, very few material properties depend directly on atomic mass. Fractional diffusion of gases is almost the only property that can be expected to effect a partial separation of a group of isotopic elements into their constituents. Whilst, to detect the non-homogeneity if it exists, the new positive ray method of Sir J. J. Thomson[10] is again almost the only one available. The examination of atmospheric neon by the method revealed the presence of atoms, in relatively small proportion, of mass 22, in addition to the known atom of mass 20. The relative proportion of the two kinds of atoms was unchanged after a prolonged fractionation of the gas by cold charcoal; but fractional diffusion showed that atmospheric neon is not homogeneous, and a partial separation, attested by a change of density, was effected by this means. No change in the spectrum corresponding with the change of density was observed, however, and the two elements appeared to be identical in all properties, except atomic weight.[11]
This accords with what has been found in the case of ionium and thorium, as regards the spectra, and in the case of the radium and thorium emanation, as regards the volatility, and indicates that isotopes will prove to be identical in these respects as they are in chemical character. At the same time, the discovery is a most dramatic extension of what has been found for the elements at one extreme of the periodic table, to the case of an element at the other extreme, and strengthens the view that the complexity of matter in general is greater than the periodic law alone reveals. Although the complexity is greater, the problem of atomic structure has been much simplified, because the generalisation gives a probable explanation of the absence of exact simple numerical relations among the atomic weights.
...
[1]Ann. Report, 1910, 285.
[2]F. Soddy, "Chemistry of the Radio-elements," 1911, p. 30.
[3]Ann. Report, 1912, 311, 321, 319. Compare also E. Marsden and R. H. Wilson, Phil. Mag., 1913, [vi], 26, 354; A., ii, 907; P. Beer and K. Fajans, Physikal. Zeitsch., 1913, 14, 947; A., ii, 907; A. B. Wood, Phil. Mag., 1913, [vi], 26, 586; A., ii, 908; K. Fajans, Physikal. Zeitsch., 14, 951; A., ii, 908; G. von Hevesy and L. von Putnoky, Physikal. Zeitsch., 1913, 14, 63; Phil. Mag., 1913, [vi], 25, 415; A., ii, 175.
[4]A. Fleck, Chem. News, 1912, 106, 128; 1913, 107, 95; T., 1913, 103, 381, 1052.
[5]Compare also W. Metzener, Ber., 1913, 46, 979; A., ii, 375.
[6]A. S. Russell, Chem. News, 1913, 107, 49; A., ii, 274; K. Fajans, Physikal. Zeitsch., 1913, 14, 131, 136; Ber., 1913, 46, 422; A., ii, 276, 277; Ber. Deut. physikal. Ges., 1913, 15, 240; A., ii, 493; Le Radium, 1913, 10, 171; A., ii, 660; F. Soddy, Chem. News, 1913, 107, 97; Jahrb. Radioaktiv. Elektronik, 1913, 10, 188; A., ii, 275.
[7]"Chemistry of the Radio-elements. Part II. The Radio-elements and the Periodic Law." By F. Soddy: Longmans, Green & Co., 1914.
[8]Ann. Report, 1912, 321.
[9]W. M. Hicks, Phil. Trans., 1913, A, 213, 323; A., ii, 810.
[10]Sir J. J. Thomson, Bakerian Lecture, Proc. Roy. Soc., 1913, 89, A, 1; A., ii, 820.
[11]F. W. Ashton [sic --CJG]: Paper communicated to the British Association, Section A., Birmingham, 1913.
 Back to the list of selected historical papers.
Back to the list of selected historical papers.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.

 Back to the list of selected historical papers.
Back to the list of selected historical papers.