The present researches had as their starting-point the facts that had come to light with regard to thorium radioactivity (Rutherford, Phil. Mag., 1900, vol. xlix, pp. 1 and 161). Besides being radioactive in the same sense as the uranium compounds, the compounds of thorium continuously emit into the surrounding atmosphere a gas which possesses the property of temporary radioactivity. This 'emanation', as it has been named, is the source of rays, which ionize gases and darken the photographic film.[1]
The most striking property of the thorium emanation is its power of exciting radioactivity on all surfaces with which it comes into contact. A substance after being exposed for some time in the presence of the emanation behaves as if it were covered with an invisible layer of an intensely active material. If the thoria is exposed in a strong electric field, the excited radioactivity is entirely confined to the negatively charged surface. In this way it is possible to concentrate the excited radioactivity on a very small area. The excited radioactivity can be removed by rubbing or by the action of acids, as, for example, sulphuric, hydrochloric, and hydrofluoric acids. If the acids be then evaporated, the radioactivity remains on the dish.
The emanating power of thorium compounds is independent of the surrounding atmosphere, and the excited activity it produces is independent of the nature of the substance on which it is manifested. These properties made it appear that both phenomena were caused by minute quantities of special kinds of matter in the radioactive state, produced by the thorium compound.
The next consideration in regard to these examples of radioactivity, is that the activity in each case diminishes regularly with the lapse of time, the intensity of radiation at each instant being proportional to the amount of energy remaining to be radiated. For the emanation a period of one minute, and for the excited activity a period of eleven hours, causes the activity to fall to half its value.
These actions-- (1) the production of radioactive material, and (2) the dissipation of its available energy by radiation--which are exhibited by thorium compounds in the secondary effects of emanating power and excited radioactivity, are in reality taking place in all manifestations of radioactivity. The constant radioactivity of the radioactive elements is the result of an equilibrium between these two opposing processes.
But the most important objection to the photographic method is that certain types of rays from radioactive substances, which ionize gases strongly, produce little if any effect on the sensitive film. In the case of uranium, these photographically inactive rays form by far the greatest part of the total radiation, and much of the previous work on uranium by the photographic method must be interpreted differently (Soddy, Proc. Chem. Soc., 1902, p. 121).
On the other hand, it is possible to compare intensities of radiation by the electrical method with greater rapidity and with an error not exceeding 1 or 2 percent. These methods are based on the property generally possessed by all radiations of the kind in question, of rendering a gas capable of discharging both positive and negative electricity. These, as will be shown, are capable of great refinement and certainty. An ordinary quadrant electrometer is capable of detecting and measuring a difference of potential of at least 10-2 volt. With special instruments, this sensitiveness may be increased a hundredfold. An average value for the capacity of the electrometer and connexions is 3 x 10-5 microfarad; and when this is charged up to 10-2 volt, a quantity of electricity corresponding to 3 x 10-13 coulomb is stored up. Now in the electrolysis of water one gram of hydrogen carries a charge of 105 coulombs. Assuming, for the sake of example, that the conduction of electricity in gases is analogous to that in liquids, this amount of electricity corresponds to the transport of a mass of 3 x 10-18 gram of hydrogen; that is, a quantity of the order of 10-12 times that detected by the balance. For a more delicate instrument, this amount would produce a large effect.
The examples of radium in pitchblende and of the thorium-excited radioactivity make it certain that comparatively large ionization effects are produced by quantities of matter beyond the range of the balance or spectroscope.
The electrometer also affords the means of recognizing and differentiating between the emanations and radiations of different chemical substances. By the rate of decay the emanation from thorium, for example, can be instantly distinguished from that produced by radium; and although a difference in the rate of decay does not of itself argue a fundamental difference of nature, the identity of the rate of decay furnishes at least strong presumption of identity of nature.
Radiations, on the other hand, can be compared by means of their penetration powers (Rutherford, Phil. Mag., 1899, vol. xlvii, p. 122). If the rays from various radioactive substances are made to pass through successive layers of aluminium foil, each additional layer of foil cuts down the radiation to a fraction of its former value, and a curve can be plotted with the thickness of metal penetrated as abscissae, and the intensity of the rays after penetration as ordinates, expressing at a glance the penetration power of the rays under examination. The curves so obtained are quite different for different radioactive substances. The radiations from uranium, radium, thorium, each give distinct and characteristic curves, whilst that of the last named again is quite different from that given by the excited radioactivity produced by the thorium emanation. It has been recently found (Rutherford and Grier, Phys. Zeit., 1902, p. 385) that thorium compounds, in addition to a type of easily absorbed Röntgen rays, non-deviable in the magnetic field, emit also rays of a very penetrating character deviable in the magnetic field. The latter are therefore similar to cathode rays, which are known to consist of material particles travelling with a velocity approaching that of light. But thorium, in comparison with uranium and radium, emits a much smaller proportion of deviable radiation. The determination of the proportion between the deviable and non-deviable rays affords a new means of investigating thorium radioactivity.
The electrometer thus supplies the study of radioactivity with methods of quantitative and qualitative investigation, and there is therefore no reason why the cause and nature of the phenomenon should not be the subject of chemical investigation.
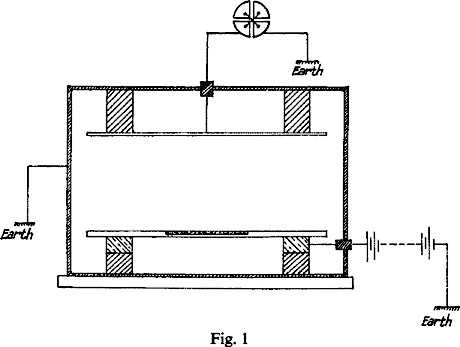
Fig. 1 shows the general arrangement. From 0.5 to 0.1 gram of the compound to be tested, reduced to fine powder, is uniformly sifted over a platinum plate 36 sq. cm. in area.

This plate was placed on a large metal plate connected to one pole of a battery of 300 volts, the other pole of which was earthed. An insulated parallel plate was placed about 6 cm. above it, and the whole apparatus enclosed in a metal box connected to earth, to prevent electrostatic disturbance. The shaded portions in the figure represented insulators. A door was made in the apparatus so that the plate could be rapidly placed in position or removed. Both pairs of quadrants are first connected to earth. On connecting the one pair with the apparatus, the deflexion of the needle from zero increases uniformly with time, and the time taken to pass over 100 divisions of the scale is taken by a stop-watch. The rate of movement is a measure of the ionization current between the plates. The ratio of the currents for different substances is a comparative measure of their radioactivity.
With this apparatus 0.5 gr. of thorium oxide produces a current of 1.1 x 10-11 amperes, which, with the electrometer used, working at average sensitiveness, corresponds to 100 divisions of the scale in 36 seconds. In certain cases a special modification of the Dolezalek electrometer was employed which is 100 times more sensitive. With this instrument the radioactivity of 1 milligram of thoria produces a measurable effect. If the substance gives off an emanation, the current between the plates increases with time. Under these conditions, when the thorium compound is exposed in thin layers with a maximum of radiating surface, all but one or two per cent of the total effect is due to the straight line radiation. Even when the effect due to the emanation has attained a maximum, this constitutes a very small fraction of the whole. This effect, however, may to a large extent be eliminated by taking the current between the electrodes immediately after the material is placed in the testing apparatus. It may be completely eliminated by passing a current of air between the electrodes to remove the emanation as fast as it is formed.
The current between the plates observed with the electrometer at first increases with the voltage, but a stage is very soon reached when there is a very small increase for a large additional voltage. A P.D. of 300 volts was sufficient to obtain the maximum current, so that all the ions reached the electrodes before any appreciable recombination occurred.
It must, however, at once be pointed out that it is difficult to make any absolute measure of radioactivity. The radiation from thorium is half absorbed by a thickness of aluminium of 0.0004 cm.; and since thorium oxide is far denser than aluminium, it is probable that the radiation in this case is confined to a surface layer only 0.0001 cm. deep. It is obvious that different preparations, each containing the same percentage of thorium but with different densities and different states of division, will not give the same intensity of radiation. In comparing two different specimens of the same compound, it is important that the final steps in their preparation should be the same in each case. As a rule absolute measurements of this kind have been avoided. It is possible, however, to trace with great accuracy the change of radioactivity of any preparation with time by leaving it undisturbed on its original plate, and comparing it with a similarly undisturbed constant comparison sample. Most of the investigations have been carried out by this method.
An examination of the penetrating power of the rays from the radioactive residue showed that the radiations emitted were in every respect identical with the ordinary thorium radiation. In another experiment the nature of the emanation from a similar intensely active thorium-free residue was submitted to examination. The rate of decay was quite indistinguishable from that of ordinary thorium emanation; that is, substances chemically free from thorium have been prepared posessing thorium radioactivity in an intense degree.
The thorium hydroxide which had been submitted to the above process was found to be less than half as radioactive as the same weight of thorium oxide. It thus appeared that a constituent responsible for the radioactivity of thorium had been obtained, which possessed distinct chemical properties and an activity of the order of at least a thousand times as great as the material from which it had been separated.
Sir William Crookes (Proc. Roy. Soc., 1900, lxvi, p. 409) succeeded in separating a radioactive constituent of great activity and distinct chemical nature from uranium, and gave the name UrX to this substance. For the present, until more is known of its real nature, it will be convenient to name the active constituent of thorium ThX, similarly. Like UrX, however, ThX does not answer to any definite analytical reactions, but makes its appearance with precipitates formed in its solution even when no question of insolubility is involved. This accords with the view that it is present in infinitesimal quantity, and possesses correspondingly great activity. Even in the case of the most active preparations, these probably are composed of some ThX associated with accidental admixtures large in proportion.
These results receive confirmation from observations made on a different method of separating ThX. The experiment was tried of washing thoria with water repeatedly, and seeing if the radioactivity was thereby affected. In this way it was found that the filtered washings, on concentration, deposited small amounts of material with an activity often of the order of a thousand times greater than that of the original sample. In one experiment, 290 grams of thoria were shaken for a long time with nine quantities, each of 2 litters of distilled water. The first washing, containing thorium sulphate present as an impurity, was rejected, the rest concentrated to different stages and filtered at each stage. One of the residues so obtained weighed 6.4 mg., and was equivalent in radioactivity to 11.3 grams of the original thoria, and was therefore no less than 1800 more radioactive. It was examined chemically, and gave, after conversion into sulphate, the characteristic reaction of thorium sulphate, being precipitated from its solution in cold water by warming. No other substance than thorium could be detected by chemical analysis, although of course the quantity was too small for a minute examination. The penetrating power of the radiation from this substance again established its identity with the ordinary thorium radiation. In another experiment, a small quantity of thoria was shaken many times with large quantities of water. In this case, the radioactivity of the residue was examined and found to be about 20 per cent less radioactive than the original sample.
The influence of Time on the activity of Thorium and ThX. The preparations employed in our previous experiments were allowed to stand over during the Christmas vacation. On examining them about three weeks later it was found that the thorium hydroxide, which had originally possessed only about 36 per cent of its normal activity, had almost completely recovered the usual value. The active residues, on the other hand, prepared by both methods, had almost completely lost their original activity. The chemical separation effected was thus not permanent in character. At this time M. Becquerel's paper (Comptes Rendus, cxxxiii, December 9, 1901, p. 977) came to hand, in which he shows that the same phenomena of recovery and decay are presented by uranium after it has been partially separated from its active constituent by chemical treatment.
A long series of observations was at once started to determine:
The normal or constant radioactivity possessed by thorium is an equilibrium value, where the rate of increase of radioactivity due to the production of fresh active material is balanced by the rate of decay of radioactivity of that already formed. It is the purpose of the present paper to substantiate and develop this hypothesis.
The active filtrate from the preparation was concentrated and made up to 100 c.c. volume. One quarter was evaporated to dryness and the ammonium nitrate expelled by ignition in a platinum dish, and the radioactivity of the residue tested at the same intervals as the hydroxide to determine the rate of decay of its activity. The comparison in this case was a standard sample of uranium oxide kept undisturbed on a metal plate, which repeated work has shown to be a perfectly constant source of radiation. The remainder of the filtrate was used for other experiments.
The following table gives an example of one of a numerous series of observations made with different preparations at different times. The maximum value obtained by the hydroxide and the original value of the ThX are taken as 100:
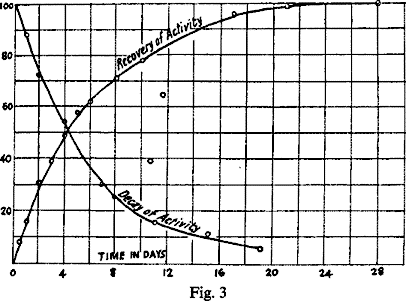
Fig. 2 shows the curves obtained by plotting the radioactivities as the ordinates, and the time in days as abscissae. Curve II. illustrates the rate of recovery of the activity of thorium, curve I. the rate of decay of the activity of ThX. It will be seen that neither of the curves is regular for the first two days. The activity of the hydroxide at first actually diminished and was at the same value after two days as when first prepared. The activity of the ThX, on the other hand, at first increases and does not begin to fall below the original value till after the lapse of two days. (compare section IX). These results cannot be ascribed to errors of measurement, for they have been regularly observed whenever similar preparations have been tested. The activity of the residue obtained from thorium oxide by the second method of washing decayed very similarly to that of ThX, as shown by the above curve.
Time in days Activity of Hydroxide Activity of ThX 0 44 100 1 37 117 2 48 100 3 54 88 4 62 72 5 68 -- 6 71 53 8 78 -- 9 -- 29.5 10 83 25.2 13 -- 15.2 15 -- 11.1 17 96.5 -- 21 99 -- 28 100 --
If for present purposes the initial periods of the curve are disregarded and the later portions only considered, it will be seen at once that the time taken for the hydroxide to recover one half of its lost activity is about equal to the time taken by the ThX to lose half its activity, viz., in each case about 4 days, and speaking generally the percentage proportion of the lost activity regained by the hydroxide over any given interval is approximately equal to the percentage proportion of the activity lost by the ThX during the same interval. If the recovery curve is produced backwards in the normal direction to cut the vertical axis, it will be seen to do so at a minimum of about 25 per cent., and the above result holds even more accurately if the recovery is assumed to start from this constant minimum, as indeed, it has been shown to do under suitable conditions (section IX, Fig. 4).
This is brought out by Fig. 3, which represents the recovery curve of thorium in which the percentage amounts of activity recovered, reckoned from this 25 per cent. minimum, are plotted as ordinates. In the same figure the decay curve after the second day is shown on the same scale.
The activity of ThX decreases very approximately in a geometrical progression with the time, i.e. if I0 represent the initial activity and It the activity after time t,
where λ is a constant and e the base of natural logarithms.
(1) It/I0 = e-λt ,
The experimental curve obtained with the hydroxide for the rate of rise of its activity from a minimum to a maximum value will therefore be approximately expressed by the equation
where I0 represents the amount of activity recovered when the maximum is reached, and It the activity recovered after time t, λ being the same constant as before.
(2) It/I0 = 1- e-λt ,
Now this last equation has been theoretically developed in other places (compare Rutherford, Phil. Mag., 1900, pp. 10 and 181) to express the rise of activity to a constant maximum of a system consisting of radiating particles in which
It therefore follows that if the initial irregularities of the curves are disregarded and the residual activity of thorium is assumed to possess a constant value, the experimental curve obtained for the recovery of activity will be explained if two processes are supposed to be taking place:
Without at first going into the difficult questions connected with the initial irregularities and the residual activity, the main result that follows from the curves given can be put to experimental test very simply. The primary conception is that the major part of the radioactivity of thorium is not due to the thorium at all, but to the presence of a non-thorium substance in minute amount which is being continuously produced.
This view, however, can be shown to be quite untenable, for upon it any precipitate capable of removing thorium completely from its solution should yield active residues similar to those obtained from ammonia. Quite the reverse, however, holds.
When thorium nitrate is precipitated by sodium or ammonium carbonate, the residue from the filtrate by evaporation and ignition is free from activity, and the thorium carbonate possesses the normal value for its activity.
The same holds true when oxalic acid is used as the precipitant. This reagent even in strongly acid solution precipitates almost all of the thorium. When the filtrate is rendered alkaline by ammonia, filtered, evaporated, and ignited, the residue obtained is inactive.
In the case where sodium phosphate is used as the precipitant in ordinary acid solution, the part that comes down is more or less free from ThX. On making the solution alkaline with ammonia, the remainder of the thorium is precipitated as phosphate, and carries with it the whole of the active constituent, so that the residue from the filtrate is again inactive.
In fact ammonia is the only reagent of those tried capable of separating ThX from thorium.
The result of Sir William Crookes with uranium, which we have confirmed with the electrical method, may be here mentioned. UrX is completely precipitated by ammonia together with uranium, and the residue obtained by the evaporation of the filtrate is quite inactive.
There can thus be no question that both ThX and UrX are distinct types of matter with definite chemical properties. Any hypothesis that attempts to account for the recovery of activity of thorium and uranium with time must of necessity start from this primary conception.
The removal of ThX was followed by measuring the activity of the residues obtained from the successive filtrates. The activity of the ThX from the first filtrate was equivalent to 4.25 grams of thoria, from the second to 0.33 gram, and from the third to 0.07 gram. It will be seen that by two precipitations practically the whole of the ThX is removed. The radioactivity of the separated hydroxide was 48 per cent. of that of the standard de-emanated sample of thoria.
Rate of Production of ThX. A quantity of thorium nitrate solution that had been freed from ThX about a month before, was again subjected to the same process. The activity of the residue from the filtrate in an experiment in which 10 grams of this nitrate had been employed was equivalent to 8.3 grams of thorium oxide. This experiment was performed on the same day as the one recorded above, in which 5 grams of new nitrate had been employed, and it will be seen that there is no difference in the activity of the filtrate in the two cases. In one month the activity of the ThX in a thorium compound again possesses its maximum value.
If a period of 24 hours is allowed to elapse between the successive precipitations, the activity of the ThX formed during that time corresponds to about one-sixth of the maximum activity of the total thorium employed. In three hours the activity of the amount produced is about one-thirtieth. The rate of production of ThX worked out from those figures well agrees with the form of the curve obtained for the recovery of activity of thorium, if the latter is taken to express the continuous production of ThX at a constant rate and the diminution of the activity of the product in geometrical progression with time.
By using the sensitive electrometer, the course of production of ThX can be followed after extremely short intervals. Working with 10 grams of thorium nitrate, the amount produced in the minimum time taken to carry out the successive precipitations is as much as can be conveniently measured. If any interval is allowed to lapse the effect is beyond the range of the instrument, unless the sensitiveness is reduced to a fraction of its ordinary value by the introduction of capacities into the system. Capacities of 0.01 and 0.02 microfarad, which reduce the sensitiveness to less than one two-hundredth of the normal, were frequently employed in dealing with these active residues.
The process of the production of ThX is continuous, and no alteration was observed in the amount produced in a given time after repeated separations. In an experiment carried out for another purpose after 23 successive precipitations extending over 9 days, the amount formed during the last interval was as far as could be judged no less than what occurred at the beginning of the process.
The phenomenon of radioactivity, by means of the electrometer as its measuring instrument, thus enables us to detect and measure changes occurring in matter after a few minutes' interval, which have never yet been detected by the balance or suspected of taking place.
Effect of conditions on the rate of decay. Since the activity of the products affords the means of measuring the amount of change, the influence of conditions on the rate of decay must be first found. It was observed that, like all other types of temporary radioactivity, the rate of decay is unaltered by any known agency. It is unaffected by ignition and chemical treatment, and the material responsible for it can be dissolved in acids and re-obtained by the evaporation of the solution, without affecting the activity. The following experiment shows that the activity decays at the same rate in solutions as in the solid state. The remainder of the solution that had been used to determine the decay curve of ThX (Fig. 2) was allowed to stand, and at the end of 12 days a second quarter was evaporated to dryness and ignited, and its activity compared with that of the first which had been left since evaporation upon its original platinum dish. The activities of the two specimens so compared with each other were the same, showing that in spite of the very different conditions the two fractions had decayed at equal rates. After 19 days a third quarter was evaporated, and the activity, now very small, was indistinguishable from that of the fraction first evaporated. Resolution of the residues after the activity had decayed does not at all regenerate it. The activity of ThX thus decays at a rate independent of the chemical and physical condition of the molecule.
Thus the rate of recovery of activity under different conditions in thorium compounds affords a direct measure of the rate of production of ThX under these conditions. The following experiments were performed:
One part of thorium hydroxide newly separated from ThX was sealed up in a vacuum obtained by a good Töpler pump, and the other part exposed to air. On comparing the samples 12 days later no difference could be detected between them either in their radioactivity or emanating power.
In the next experiment a quantity of hydroxide freed from ThX was divided into two equal parts; one was exposed for 20 hours to the heat of a Bunsen burner in a platinum crucible, and then compared with the other. No difference in the activities was observed. In a second experiment one half was ignited for 20 minutes on the blast, and then compared with the other with the same result. The difference of temperature and the conversion of thorium hydroxide into oxide thus exercised no influence on the activity.
Some experiments that were designed to test in as drastic a manner as possible the effect of the chemical condition of the molecule on the rate of production of ThX brought to light small differences, but these are almost certainly to be accounted for in another way. It will be shown later (section IX) that about 21 per cent of the normal radioactivity of thorium oxide under ordinary conditions consists of a secondary activity excited on the mass of the material. This portion is of course a variable, and since it is divided among the total amount of matter present, the conditions of aggregation, etc., will affect the value of this part. This effect of excited radioactivity in thorium makes a certain answer to the question difficult, and on this account the conclusion that the rate of production of ThX is independent of the molecular conditions is not final. The following experiment, however, makes it extremely probable.
A quantity of thorium nitrate as obtained from the maker was converted into oxide in a platinum crucible by treatment with sulphuric acid and ignition to a white heat. The de-emanated oxide so obtained was spread on a plate, and any change in radioactivity with time, which under these circumstances could certainly be detected, was looked for during the first week from preparation. None whatever was observed, whereas if the rate of production of ThX in thorium nitrate is different from that in the oxide, the equilibrium point, at which the decay and increase of activity balance each other, will be altered in consequence. There should have therefore occurred a logarithmic rise or fall from the old to the new value. As, however, the radioactivity remained constant, it appears very probable that the changes involved are independent of the molecular condition.
It will be seen that the assumption is here made that the proportion of excited radioactivity in the two compounds is the same, and for this reason compounds were chosen which possess but low emanating power. (Compare section IX, last paragraph.)
Uranium is a far simpler example of a radioactive element than thorium, as the phenomena of excited radioactivity and emanating power are here absent. The separation of UrX and the recovery of the activity of the uranium with time appear, however, analogous to these processes in thorium, and the rate of recovery and decay of uranium activity are at present under investigation. It is proposed to test the influence of conditions on the rate of change more thoroughly in the case of uranium, as here secondary changes do not interfere.
The nature of the process becomes clear in the light of the foregoing results. The material constituent responsible for the radioactivity, when it is separated from the thorium which produces it, then behaves in the same way as the other types of radioactivity cited. Its activity decays geometrically with the time, and the rate of decay is independent of the molecular conditions. The normal radioactivity is, however, maintained at a constant value by a chemical change which produces fresh radioactive material at a rate also independent of the conditions. The energy required to maintain the radiations will be accounted for if we suppose that the energy of the system after the change has occurred is less than it was before.
The work of Crookes and Becquerel on the separation of UrX and the recovery of the activity of the uranium with time, makes it appear extremely probable that the same explanation holds true for this element. The work of M. and Mme. Curie, the discoverers of radium, goes to show that this body easily suffers a temporary decrease of its activity by chemical treatment, the normal value being regained after the lapse of time, and this can be well interpreted on the new view. All known types of radioactivity can thus be brought under the same category.
A study of the curves (Fig. 2) shows that in each case a double action is probably at work. It may be supposed that the normal decay and recovery are taking place, but are being masked by a simultaneous rise and decay from other causes. From what is known of thorium radioactivity, it was surmised that an action might be taking place similar to that effected by the emanation of excited radioactivity on surrounding inactive matter. It will be shown later that the ThX, and not thorium, is the cause of the emanating power of the thorium compounds. On this view, the residual activity of thorium might consist in whole or in part of a secondary or excited radioactivity produced on the whole mass of the thorium compound by its association with the ThX. The drop in the recovery curve on this view would be due to the decay of this excited radioactivity proceeding simultaneously with, and at first reversing, the effect of the regeneration of ThX. The rise of the decay curve would be the increase due to the ThX exciting activity on the matter with which it is associated, the increase from this cause being greater than the decrease due to the decay of the activity of the ThX. It is easy to put this hypothesis to experimental test. If the ThX is removed from the thorium as soon as it is formed over a sufficient period, the former will be prevented from exciting activity on the latter, and that already excited will decay spontaneously. The experiment was therefore performed. A quantity of nitrate was precipitated as hydroxide in the usual way to remove ThX, the precipitate redissolved in nitric acid, and again precipitated after a certain interval. From time to time a portion of the hydroxide was removed and its radioactivity tested. In this way the thorium was precipitated in all 23 times in a period of 9 days, and the radioactivity reduced to a constant minimum. The following table shows the results:
Activity of hydroxide
per centAfter first precipitation 46 After precipitations at three intervals of 24 hours 39 At three more intervals each of 24 hours, and three more each of 8 hours 22 At three more each of 8 hours 24 At six more each of 4 hours 25
The constant minimum thus attained--about 25 per cent of the original activity--is thus about 21 per cent below that obtained by two successive precipitations without interval, which has been shown to remove all the ThX separable by the process. The rate of recovery of this 23 times precipitated hydroxide was then measured (Fig. 4). It will be seen that it is now quite normal, and the initial drop characteristic of the ordinary curve is quite absent. It is in fact almost identical with the ordinary curve (Fig. 2) that has been produced back to cut the vertical axis, and there is thus no doubt that there is a residual activity of thorium unconnected apparently with ThX, and constituting about one fourth of the whole.
The decay curves of several of the fractions of ThX separated in this experiment after varying intervals of time were taken for the first few days. All of them showed the initial rise of about 15 per cent at the end of 18 hours, and then a normal decay to zero. The position is thus proved that the initial irregularities are caused by the secondary radiation excited by ThX upon the surrounding matter. By suitably choosing the conditions the recovery curve can be made to rise normally from a constant minimum, and the decay curve be shown to consist of two curves, the first, the rate of production of excited radioactivity, and the second, the rate of decay of the activity as a whole.
So far nothing has been stated as to whether the excited radioactivity which contributes about 21 per cent of the total activity of thorium is the same or different from the known type produced by the thorium emanation. All that has been assumed is that it should follow the same general law, i.e. the effect will increase with the time of action of the exciting cause, and decrease with time after the cause is removed. If the rate of rise of the excited activity be worked out from the curves given (Fig. 5) it will be found to agree with that of the ordinary excited activity, i.e. it rises to half value in about 12 hours. Curve I is the observed decay curve for ThX; curve II is the theoretical curve, assuming that it decreases geometrically with time and falls to half value in four days. Curve III is obtained by plotting the difference between these two and, therefore constitutes the curve of excited activity. Curve IV is the experimental curve obtained for the rise of the excited radioactivity from the thorium emanation when the exciting cause is constant. But the exciting cause (ThX) in the present case is not constant, but is itself falling to half value in 4 days, and hence the difference curve, at first almost on the other, drops away from it as time goes on, and finally decays to zero. There is thus no reason to doubt that the effect is the same as that produced by the thorium emanation, which is itself a secondary effect of ThX. Curve III (Fig. 2) represents a similar difference curve for the decay of excited activity, plotted from the recovery curve of thorium.
Since this effect of excited activity is caused by the emanation, it seemed reasonable to suppose that it will be greater, the less the emanation succeeds in escaping in the radioactive state, and therefore that de-emanated compounds should possess a greater proportion of excited radioactivity than those with high emanating power. This conclusion was tested by converting a specimen of thorium carbonate with an emanating power five times that of ordinary thoria, into oxide and de-emanating by intense ignition. The energy that had escaped in the form of emanation is now, all but a few per cent, prevented from escaping. The radioactivity of the oxide so prepared rose in the first three days about thirty per cent of its original amount, and there thus seem to be grounds for the view that the excited radioactivity will contribute a much greater effect in a non-emanating thorium compound than in one possessing great emanating power.
Additional confirmation of this view is to be found in the nature of the radiations emitted by the two classes of compounds (section XI).
Secondly, if the change which gives rise to ThX produced a second type of matter at the same time, i.e. if it is of the type of a decomposition rather than a depolymerization, the second type would also in all probability be radioactive, and would cause the residual activity. On this view the second type of matter should also be amenable to separation by chemical means, although it is certain from the failure of the methods already tried that it resembles thorium much more closely than ThX. But until it is separated from the thorium producing it, its activity will not decay spontaneously. Thus what has already been shown to hold for ThX will be true for the second constituent if methods are found to remove it from the thorium.
It has been shown (Soddy, loc. cit.) that uranium also possesses a non-separable radioactivity extremely analogous to that possessed by thorium, and whatever view is taken of the one will in all probability hold also for the other. This consideration makes the second hypothesis, that the residual activity is caused by a second non-thorium type of matter produced in the original change, the more probable of the two.
Similar difficulties stand in the way of an answer to the second question, whether the nature of the radiations is affected by chemical treatment, for it has been experimentally observed that the penetrating power of these radiations decreases with the thickness of material traversed. The character of the radiations from ThX and thorium have, however, been compared by the method of penetration power. A large number of comparisons justifies the view that the character of thorium radioactivity is unaltered by chemical treatment and the separation of ThX, although the different types are unequally distributed among the separated products.
Determinations of the proportion of rays deviable by the magnetic field in thorium and ThX throws fresh light on the question. The general result is that ThX gives out both deviable and non-deviable rays, and the same applies to the excited activity produced by ThX. But in the experiment in which the excited radiation was allowed to spontaneously decay, by removing ThX as formed, the thorium compound obtained after 23 precipitations was found to be quite free from deviable radiation. This is one of the most striking resemblances between the non-separable radioactivities of uranium and thorium, and warrants the question whether the primary radiation of ThX is not, like that of UrX, composed entirely of cathode rays. There is, however, no means of deciding this point owing to the excited radiation which always accompanies the primary radiation of ThX, and which itself comprises both types of rays.
Finally, it may be mentioned that the proportion of deviable and non-deviable radiation is different for different compounds of thorium. The nitrate and ignited oxide, compounds which hardly possess any emanating power, have a higher proportion of deviable radiation than compounds with great emanating power. This is indirect evidence of the correctness of the view already put forward (section IX), that when the emanation is prevented from escaping it augments the proportion of excited radioactivity of the compound.
The ThX further possesses the property of exciting radioactivity on surrounding inactive matter, and about 21 per cent. of the total activity under ordinary circumstances is derived from this source. Its rate of decay and other considerations make it appear probable that it is the same as the excited radioactivity produced by the thorium emanation, which is in turn produced by ThX . There is evidence that, if from any cause the emanation is prevented from escaping in the radioactive state, the energy of its radiation goes to augment the proportion of excited radioactivity in the compound.
Thorium can be freed by suitable means from both ThX and the excited radioactivity which the latter produces, and then possesses an activity about 25 per cent. of its original value, below which it has not been reduced. This residual radiation consists entirely of rays non-deviable by the magnetic field, whereas the other two components comprise both deviable and non-deviable radiation. Most probably this residual activity is caused by a second non-thorium type of matter produced in the same change as ThX, and it should therefore prove possible to separate it by chemical methods.
All the most prominent workers in this subject are agreed in considering radioactivity an atomic phenomenon. M. and Mme. Curie, the pioneers in the chemistry of the subject, have recently put forward their views (Comptes Rendus, cxxxiv, 1902, p. 85). They state that this idea underlies their whole work from the beginning and created their methods of research. M. Becquerel, the original discoverer of the property for uranium, in his announcement of the recovery of the activity of the same element after the active constituent had been removed by chemical treatment, points out the significance of the fact that uranium is giving out cathode-rays. These, according to the hypothesis of Sir William Crookes and Prof. J. J. Thomson, are material particles of mass one thousandth of the hydrogen atom.
Since, therefore, radioactivity is at once an atomic phenomenon and accompanied by chemical changes in which new types of matter are produced, these changes must be occurring within the atom, and the radioactive elements must be undergoing spontaneous transformation. The results that have so far been obtained, which indicate that the velocity of the reaction is unaffected by the conditions, make it clear that the changes in question are different in character from any that have been before dealt with in chemistry. It is apparent that we are dealing with phenomena outside the sphere of known atomic forces. Radioactivity may therefore be considered as a manifestation of subatomic chemical change.
The changes brought to knowledge by radioactivity, although undeniably material and chemical in nature, are of a different order of magnitude from any that have before been dealt with in chemistry. The course of the production of new matter which can be recognized by the electrometer, by means of the property of radioactivity, after the lapse of a few hours or even minutes, might conceivably require geological epochs to attain to quantities recognized by the balance. However, the well-defined chemical properties of both ThX and UrX are not in accordance with the view that the actual amounts involved are of this extreme order of minuteness. On the other hand, the existence of radioactive elements at all in the earth's crust is an à priori argument against the magnitude of the change being anything but small.
Radioactivity as a new property of matter capable of exact quantitative determination thus possesses an interest apart from the peculiar properties and powers which the radiations themselves exhibit. Mme. Curie, who isolated from pitchblende a new substance, radium, which possessed distinct chemical properties and spectroscopic lines, used the property as a means of chemical analysis. An exact parallel is to be found in Bunsen's discovery and separation of caesium and rubidium by means of the spectroscope.
The present results show that radioactivity can also be used to follow chemical changes occurring in matter. The properties of matter that fulfil the necessary conditions for the study of chemical change without disturbance to the reacting system are few in number. It seems not unreasonable to hope, in the light of the foregoing results, that radioactivity, being such a property, affords the means of obtaining information of the processes occurring within the chemical atom, in the same way as the rotation of the plane of polarization and other physical properties have been used in chemistry for the investigation of the course of molecular change.
 Back to the list of selected historical papers.
Back to the list of selected historical papers. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.