| C4H5 | 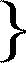
| |
| C4H5 | N,C4H5O . | |
| C4H5 |
The preceding researches show that the action of the bromides and iodides of the alcohol radicals upon ammonia, gives rise to the formation of not less than four distinct groups of organic bases. Of these the members of three groups, corresponding to ammonia (H3N), are volatile, while those of the fourth, corresponding to oxide of ammonium (H4NO), cannot be volatilized without decomposition. The facility with which the members of this last class arise from those of the preceding ones, and the readiness with which their reconversion is effected, renders the former group as it were the connecting link between the volatile and the non-volatile organic alkaloids.
...
In conclusion, it may not be out of place to consider how far the preceding researches affect the received views regarding the constitution of the ammonia-salts. Without reproducing all the arguments brought forward by the supporters of various theories, we may remember that, irrespective of the impossibility of isolating ammonium itself, the instability of its oxide has been advanced as one of the most important objections against the assumption of the ammonium-theory as originally suggested by AMPÈRE, and subsequently elaborated by BERZELIUS. It deserves to be noticed that BERZELIUS expressly states that he considers the solution of ammonia-gas in water as a solution of the hydrated oxide of ammonium.
This idea, which is but a logical conclusion from the generalization of the facts, is discountenanced to a certain extent by the chemical and physical character of this solution. Everybody knows that, even at the common temperature, this liquid splits again into water and ammonia, while it still exhibits the character of the latter in so marked a manner, as almost to preclude the idea that it had undergone as essential a change as the transformation into oxide of ammonium necessarily must be. Under these circumstances, some interest is attached to the discovery of a series of compound bases, corresponding in their composition to hydrated oxide of ammonium, from which they differ only by containing methyl, ethyl, and amyl in the place of hydrogen, and exhibiting a deportment which agrees much better with the anticipated character of such compounds as suggested by analogy. Here we find a very marked difference between the properties of the compound ammonia, and those of the ammonium-oxide belonging to it; in the latter, we observe no longer any feature which could possibly betray the presence of the former; all their habits, volatility, odour, taste, &c. are totally changed; there is a difference between the two groups which is not inferior to that between ammonia and potassia. The solutions of the new oxides may be boiled for hours without the slightest quantity of the corresponding ammonia being disengaged; several of these oxides, containing more or less water of constitution or crystallization, may actually be obtained in the dry state. It is evident that the arguments mentioned above, as adduced in refutation of the ammonium-theory, cannot well be raised against the compound ammoniums. But who could deny the parallelism of these substances with the Berzelian type--with the oxide of ammonium?
Again, many have found it difficult to conceive, that in the combination of ammonia with hydrochloric or hydrobromic acid, the hydrogen of the latter should leave the chlorine and bromine, for which it is known to possess so powerful and affinity, in order to unite with ammonia converting it into ammonium. And they were the less inclined to admit of such a disposition of the elements, as every day's experience showed that the alleged chloride or bromide of ammonium was incapable of exchanging oxygen for chlorine or bromine, without losing the additional equivalent of hydrogen again in the form of water. In other terms, the decomposition of sal-ammoniac, by lime, into chloride of calcium, ammonia-gas and water, induced them to consider this salt as a compound of ammonia and hydrochloric acid; for in the conception of the ammonium-theory we should have to assume in this decomposition two consecutive changes, the transformation of the chloride into oxide, and the subsequent splitting of the latter into ammoniacal gas and water. I readily admit that the latter view is less simple, but I am inclined to think that this slight inconvenience is altogether overruled by the general advantage of the ammonium-theory, especially for the purposes of instruction; by the facility with which it accounts for all phenomena of transposition and substitution, and by the simple explanation it gives of the isomorphism of the potassium- and ammonium-compounds, which will always be the firmest foundation of this theory. On the other hand, we have to enquire which of the two views comes nearest the truth, and here a comparative consideration of the deportment exhibited by the compound ammoniums may be of some interest. In many respects their properties are more clearly pronounced; and their behaviour is explicit and unequivocal in those very points in which the typical ammonium leaves room for speculation. In the combination of triethylamine with bromide or iodide of ethyl, it is no longer a matter of doubt whether the ethyl leaves the iodine in order to unite more intimately with the triethylamine, for we see that the new iodide thus produced is capable of exchanging its iodine for oxygen without the newly-formed oxide suffering immediate decomposition, as is the case with oxide of ammonium. On the contrary, we find this new oxide endowed with remarkable stability; although under the influence of heat it is liable to the same change which befalls the oxide of ammonium, its corresponding ammonia being reproduced. Here then, in the decomposition of iodide of triethylammonium by metallic oxides, we are obliged by irresistible evidence to acknowledge those very two stages, the assumption of which in the analogous change of iodide of ammonium appeared to us deficient in simplicity and probability.
The conception of ammonium does not in any way imply the notion that the different hydrogen-atoms united with nitrogen in the molecule of the compound metal, retain their positions in the molecular system with equal persistency. We are forced by unequivocal facts to admit that the fourth atom of hydrogen is in a peculiar state of mobility, and it is on the facility with which this fourth atom is dislodged from its position that one of the foundations of the ammonia-theory rests. In the compound ammoniums the mobility of the fourth atom of hydrogen, or the hydrocarbon replacing it, still prevails, although less so than in the type itself. The decomposition of the ammonium bases under the influence of heat is particularly instructive in this respect; oxide of tetraethylammonium loses the fourth equivalent of ethyl in the form of olefiant gas and water; and this deportment might be graphically indicated by writing the formula of this compound in accordance with the ammonia theory, namely, thus--
The iodide accordingly would be represented by the formula
C4H5 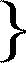
C4H5 N,C4H5O . C4H5
an expression which is moreover in perfect harmony with the mode in which this compound is produced, namely, by the direct union of iodide of ethyl with triethylamine.
C4H5 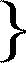
C4H5 N,C4H5I , C4H5
But now we combine the triethylamine with iodide of amyl, whereby the iodide
is formed, which, as we have seen in the preceding pages, may be converted without difficulty into the corresponding oxide; this oxide however, cannot possibly be considered as
C4H5 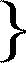
C4H5 N,C10H11I , C4H5
for the disengagement of olefiant gas under the influence of heat proves to us that it is an ethyl-atom which in the compound occupies the supplemental position, if I may so call it, as represented in the formula
C4H5 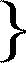
C4H5 N,C10H11O , C4H5
and that the iodide, which is not likely to differ in its constitution from the oxide, has likewise to be represented by the formula
C4H5 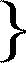
C4H5 N,C4H5O , C10H11
C4H5 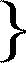
C4H5 N,C4H5I . C10H11
The preceding considerations clearly show, that, whatever the actual disposition of the molecules in ammonium or its congeners may be, the atoms rearrange themselves whenever the fourth equivalent of hydrogen, or of its substitute, joins the compound.
This re-arrangement, so evident in the ammonium-bases, containing various hydrocarbons, may be traced moreover in the lower ethyl-bases in a very obvious manner. For as long as there is any basic hydrogen present in the ammonia skeleton, this hydrogen assumes what I have previously called the supplemental position, whenever the ammonia passes into the state of ammonium by the accession of a radical. Bromide of ethylammonium formed by the combination of ammonia with bromide of ethyl, when decomposed by a metallic oxide, yields ethylammonia, water and a metallic bromide, the oxide of ethylammonium formed in the first instance being decomposed like oxide of ammonium itself. It is this very transposition which we are in the habit of representing by the equation
H3N + C4H5Br = C4H5,H2N,HBr .
...
In the preceding pages I have stated some of the reasons which induced me to adopt the idea of an ammonium for the new class of compounds which I have had the honour to place before the Royal Society in the present memoir. I need scarcely mention, that such a step involves as a matter of necessity the assumption of a similar view for all the lower bases which form part of this investigation. It would be inconsistent to speak any longer of hydrochlorate of ethylamine, of hydrobromate of diethylamine, &c.; these salts have henceforward to be called chloride of ehylammonium, bromide of diethylammonium, &c., these compounds being nothing but intermediate substitution-terms between the type and the last derivative. On considering the various chlorides from this point of view, we arrive at the following series:--
chloride of ammonium H4 N Cl . chloride of ethylammonium H3 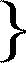
C4H5 N Cl chloride of diethylammonium H2 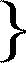
(C4H5)2 N Cl chloride of triethylammonium H 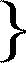
(C4H5)3 N Cl chloride of tetraethylammonium (C4H5)4 N Cl .
 Back to the list of selected historical papers.
Back to the list of selected historical papers. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.