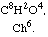
In a memoir which I had the honor of communicating to the Academy some time ago I showed that chlorine decomposes acetic acid under the influence of solar light and that it gives rise to a new acid which I have named chloroacetic acid.
I expressed on that occasion the opinion that acetic acid and chloroacetic acid belong to the same chemical type, A8B8C4, one being represented by C8H8O4 and the other by
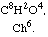
I have attempted to generalize this point of view and to show how these types may serve to group these organic substances in well-characterized genera.
As soon as he was acquainted with my memoir, M. Berzelius, who denies the theory of substitutions, published a refutation of the views which I gave there. This illustrious scientist regards acetic acid and chloracetic acid as very different from one another because they have not the same density, boiling point, nor odor, etc.
M. Berzelius has certainly not understood what I have called the fundamental properties of substances, for long ago I knew that in replacing the hydrogen in a compound by chlorine the compound is rendered more dense and less volatile and that at the same time its vapor density is increased.
Moreover, it is perfectly clear to me that the objections advanced by M. Berzelius do not apply at all to the views which I actually intended to express.
However, to prevent all misunderstanding I will attempt to state my idea precisely by means of an example.
By treating chloracetic acid with any alkali I have obtained a very remarkable reaction. The acid is converted into two new substances, namely carbonic acid, which is combined with the alkali, and chloroform, which is liberated. We have thus
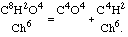
I was convinced, and I announced it after a fashion in my memoir, that acetic acid would give an analogous reaction: that is to say, that under the influence of an excess of base it would change into carbonic acid and a hydrocarbon which should have C4H8 for a formula.
After several inevitable trials I have completely succeeded in producing this remarkable reaction.
It sufficed to mix 10 grams of crystallized acetate of soda with 30 or 40 grams of caustic baryta and to heat the mixture very gently in a retort to effect the transformation of acetic acid into carbonic acid and a gas which has the formula C4H8.
Nothing could be more distinct than this decomposition: the residue remained perfectly white; not the least trace of oil or pyroacetic spirit, not the least vapor, other than the water which accompanied the gas, was evolved.
Here is the eudiometric analysis of this gas:
| Gas | 32 | 31 | 30 |
| Oxygen | 91 | 86 | 84 |
| Residue after the explosion | 59 | 55.5 | 54 |
| The potash left | 27 | 25.5 | 24 |
| We have then: | |||
| Carbon | 32 | 30 | 30 |
| Hydrogen | 64 | 61 | 60 |
But such is exactly the composition of a gas which chemists have never known how to produce; I mean marsh gas.
One cannot refrain from remarking on these connections which are manifest between marsh gas produced by the spontaneous decomposition of vegetable matters and the marsh gas arising from the final decomposition of acetic acid which, itself, was produced by the dry distillation of wood.
I propose to make a complete study of this gas and to make a complete examination of reactions analogous to that which furnished it.
For the present I confine myself to establishing in an exact manner that the gas C4H8, corresponding to chloroform
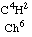
according to the theory of substitution, was produced by acetic acid, just as chloroform was by chloroacetic acid.
This is to say that acetic and chloracetic acids possess the same fundamental chemical properties, as I had established, and belong to the same organic type.
 Back to the list of selected historical papers.
Back to the list of selected historical papers. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.