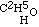 , and the potassium compound is
, and the potassium compound is 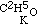 ; and by acting upon this by iodide of aethyle, we have
; and by acting upon this by iodide of aethyle, we have
When sulphuric acid is brought in contact with alcohol under certain circumstances, a new arrangement is effected in the elements of the alcohol, which divide into two groups, forming aether and water. Now it is well known that the process by which this change is effected may be represented in two ways, the difference of which consists in their respectively selecting for starting-point a different view of the constitution of alcohol. According to the one view, an atom of alcohol weighs 23, and is made up of C2H6O; so that to form aether, two atoms of it are needed, one of which takes C2H4 from the other, setting free the water with which these elements were combined; whereas, according to the other view, alcohol weighs 46, and contains ether and water. These are not the only points of difference which are urged; but they are the most real and tangible, and their consideration is sufficient for our present purpose. If by any direct fact we could decide which of these two expressions is the correct one, the ground would be clear for an examination of the process of aetherification itself. In order to show more clearly the true meaning of the facts I have to adduce on this point, I will bring them before you in the order in which they arose.
My object in commencing the experiments was to obtain new alcohols by substituting carburetted hydrogen for hydrogen in a known alcohol. With this view I had recourse to an expedient, which may render valuable services on similar occasions. It consisted in replacing the hydrogen first by potassium and acting upon the compound thus formed by the chloride or iodide of the carburetted hydrogen which was to be introduced in the place of that hydrogen. I commenced with common alcohol, which, after careful purification, was saturated with potassium, and as soon as the action had ceased, mixed with a portion of iodide of aethyle equivalent to the potassium used. Iodide of potassium was readily formed on the application of a gentle heat, and the desired substitution was effected; but, to my astonishment, the compound thus formed had none of the properties of an alcohol--it was nothing else than common aether, C4H10O.
Now this result at once struck me as being inconsistent with the higher formula for alcohol; for if that body contained twice as many atoms of oxygen as are in aether, I ought clearly to have obtained a product containing twice as much oxygen as aether does. The alternative was evident; for having obtained aether by substituting C2H5 for H in alcohol, the relative composition of the two bodies is represented by expressing that fact in our formula. Thus alcohol is 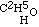 , and the potassium compound is
, and the potassium compound is 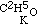 ; and by acting upon this by iodide of aethyle, we have
; and by acting upon this by iodide of aethyle, we have
Of course the proportion between the two bodies is the only point upon which I here enter, and the same reasoning would be applicable to any multiple of the formulae assumed. Some chemists may perhaps prefer doubling them in order to avoid the use of atoms of hydrogen, potassium, &c.; but I have not felt myself justified in doing so, because that would involve doubling the usual formula for water; for, as I will presently show, water is formed in aetherification by replacing the carburetted hydrogen of alcohol by hydrogen, which, of course, obliges us to assume the same unity of oxygen in both. Alcohol is therefore water in which half the hydrogen is replaced by carburetted hydrogen, and aether is water in which both atoms of hydrogen are replaced by carburetted hydrogen: thus,.
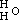 ,
, 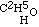 ,
, 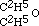 .
.This formation of aether might however be explained after a fashion by the other theory--by supposing the potassium compound to contain aether and potash, which separate during the action of the iodide of aethyle; so that half the aether obtained would have been contained in that compound, and the other half formed by double decomposition between potash and iodide of aethyle: thus--
But although the insufficiency of this explanation becomes evident on a little refection, I devised a further and more tangible method of arriving at a conclusion. It consisted in acting upon the potassium compound by iodide of methyle, in which case I should, if that compound were aether and potash, obtain a mixture of aether and oxide of methyle; whereas in the contrary case I should obtain a body of the composition C3H8O. Now this substance I obtained, and neither aether nor oxide of methyle..
In this experiment the two theories cross one another, and must lead to different results; for it is evident that, in the first-mentioned decomposition by which aether was formed, the only difficulty in explaining the process decisively consisted in our inability to prove that the carburetted hydrogen introduced instead of the hydrogen did not have in the product an atom of oxygen to itself, but that, on the contrary, it was coupled with the carburetted hydrogen already contained in the alcohol--the two in combination with one atom of oxygen. It is clear that if alcohol contain aether and water, and the carburetted hydrogen in my first experiment formed a second atom of aether by taking the place of the hydrogen of this water, that the process being the same in the second experiment, we should then have obtained two aethers. Whereas if the formation of aether from alcohol be effected by synthesis, a new carburetted hydrogen being added to the one already contained in the alcohol, we ought to obtain the new intermediate aether which I obtained.
The complete description of this remarkable body and of its decompositions, will form the subject of a future paper. I will now merely state that its boiling point is a little above 10° Cent.; it is possessed of a very peculiar smell, distinctly different from that of common aether; and, like that body, it is only slightly soluble in water. It is not acted upon by the alkali-metals at the common atmospheric temperature.
By acting upon the potassium-alcohol in like manner by iodide of amyle, I effected a similar substitution of the elements of that carburetted hydrogen in the place of the hydrogen of alcohol, and obtained an aether boiling at 111°C., having the composition C7H16O. There is some reason to believe that this body is the same which Balard obtained by decomposition of chloride of amyle by an alcoholic solution of hydrated potash, and which that distinguished chemist took for oxide of amyle.
From the perfect analogy of properties between the known terms of the alcoholic series, it was to be expected that similar substitutions might be effected in the others; and I have verified this by experiment. Of course the formulae of the other alcohols must be reduced to half, for the same reasons as that of common alcohol. Methylic alcohol is therefore expressed by the formula 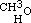 , as common alcohol is
, as common alcohol is 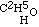 ; and in the same manner amylic alcohol is
; and in the same manner amylic alcohol is 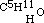 , and the same of the higher ones. In conformity to this fact, we must be able to obtain the same intermediate aethers by replacing hydrogen in these alcohols (methylic and amylic) by the carburetted hydrogen of iodide of aethyle, as by the inverse process described above. This I have verified in the case of the three-carbon aether, which may be obtained indifferently by replacing one-fourth of the hydrogen of methylic alcohol by C2H5, or by replacing one-sixth of the hydrogen of common alcohol by CH3. Its rational formula is therefore
, and the same of the higher ones. In conformity to this fact, we must be able to obtain the same intermediate aethers by replacing hydrogen in these alcohols (methylic and amylic) by the carburetted hydrogen of iodide of aethyle, as by the inverse process described above. This I have verified in the case of the three-carbon aether, which may be obtained indifferently by replacing one-fourth of the hydrogen of methylic alcohol by C2H5, or by replacing one-sixth of the hydrogen of common alcohol by CH3. Its rational formula is therefore 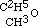 .
.
By acting upon the compound 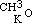 by iodide of amyle, I obtained a third aetheral compound, of which the formula is
by iodide of amyle, I obtained a third aetheral compound, of which the formula is 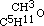 . This is evidently the only one of the three new aethers, which, containing an even number of carbon atoms, might be conceived to have been formed from one alcohol; but when treated with monobasic acids, as hydrochloric, it cannot be expected to act in the same manner as its homogeneous isomeric, the aether
. This is evidently the only one of the three new aethers, which, containing an even number of carbon atoms, might be conceived to have been formed from one alcohol; but when treated with monobasic acids, as hydrochloric, it cannot be expected to act in the same manner as its homogeneous isomeric, the aether 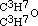 of the three-carbon alcohol
of the three-carbon alcohol 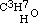 ; but of this I will give an exact account in the paper above alluded to.
; but of this I will give an exact account in the paper above alluded to.
My task is now to explain the process of aetherification by the action of sulphuric acid (SO4H2) upon alcohol; and in order to accomplish that, I must show the connexion between those substances and the reagents used in the above-described experiments. With this view, I have merely to add to the above facts the acknowledged analogy of the simple and compound radicals in their compounds. I must first show how a substance analogous to my iodide of aethyle is formed, and then how by double decomposition with alcohol it produces aether. This is very easy; for sulphovinic acid is strictly analogous to iodide of aethyle plus iodide of hydrogen, which we should obtain by replacing SO4 in its formula by an equivalent of iodine; and in order to represent the formation of this sulphovinic acid, which is well known to precede that of aether, the simplest mode is at the same time the one most free from hypothesis; it consists in stating the fact, that sulphuric acid and alcohol are transformed into sulphovinic acid and water, by half the hydrogen of the former changing places with the carburetted hydrogen of the latter: thus--
Now from this point it is clear that the process is the same as in the decompositions above described; for by this sulphovinic acid coming in contact with an atom of alcohol, it reacts in the same manner as the iodide did, forming of course sulphuric acid and aether:
The sulphuric acid thus reproduced comes again in contact with alcohol, forming sulphovinic acid, which reacts as before; and so the process goes on continuously, as found in practice.
We thus see that the formation of aether from alcohol is neither a process of simple separation, nor one of mere synthesis; but that it consists in the substitution of one molecule for another, and is effected by double decomposition between two compounds. I therefore admit the contact theory, inasmuch as I acknowledge the circumstance of contact as a necessary condition of the reaction of the molecules upon one another. By reducing the formulae of the alcohols to one atom of oxygen, I also retain the equality of volumes which the contact theory insists upon between the vapours of these bodies and their aethers, so that aether truly contains the elements of olefiant gas in addition to those of alcohol in one atom. But, on the other hand, I attach equal importance to all the essential facts of the chemical theory, and rest my explanation of the process as much upon them as upon those of the contact theory; for, one-sixth of the hydrogen in alcohol truly exhibits different reactions from the remaining five, and must therefore be contained in that compound in a different manner from them; and the alternate formation and decomposition of sulphovinic acid is to me, as to the partisans of the chemical theory, the key to explaining the process of aetherification.
Innovations in science frequently gain ground only by displacing the conceptions which preceded them, and which served more or less directly as their foundation; but, if the view which I have here presented be considered a step in our understanding of the subject, I must beg leave to disclaim for it the title of innovation; for my conclusion consists in establishing the connexion and showing the compatibility of views which have hitherto been considered contrary; and the best possible justification of the eminent philosophers who advocated either one of the two contending theories, is thus afforded by my reconciling their arguments with those of their equally illustrious opponents.
The human mind is only capable of understanding complicated phenomena when prepared by the study of simpler ones; and one of the most remarkable illustrations of this necessary order is afforded by the preparation of dynamical laws by the consideration of statical facts. In statics we consider phenomena in a state of rest, while in dynamics we study their change; and this distinction has been concisely stated by saying that the transition from the statical to the dynamical point of view, consists in superadding the consideration of time to that of space.
...
The lecturer submitted that the definite compounds hitherto exclusively acknowledged and studied by chemists, are in truth only exceptionally simple cases of combination, and that the consideration of chemists is only limited to them, because the atomic theory is as yet purely statical. The atomic theory has hitherto been tacitly connected with an unsafe and unjustifiable hypothesis, namely, that the atoms are in a state of rest; the dynamics of chemistry will study the degree and kind of motion which atoms possess, and reduce to this one fact the various phenomena of change, which are now attributed to occult forces. But although it will probably be generally used in connexion with the atomic theory, the fact of motion is independent of any particular theory; and however the properties of matter may be conceived, it will remain true, that a change of place among the representatives or possessors of these properties, is constantly going on, which produces the phenomena of chemical combination.
...
There are many prima facie evidences that time is necessary for chemical action--but this fact, although it has been noticed, has not as yet entered into the explanation of phenomena.
The one instance in which a certain regular motion of the constituents of a mixture was first proved, is the process of etherification, of which the anomalous character has long since attracted the attention and study of many of the most eminent chemists, and has given rise to various theories which respectively represented part of the phenomena.
...
Having proved by a direct experiment that the formation of ether from alcohol is effected by substituting ethyl (C2H5) for one sixth of the hydrogen of that body, the process of etherification by sulphuric acid was explained by a diagram, on which half the hydrogen in sulphuric acid was shown to change places with its analogue ethyl in alcohol; and that the peculiarity of the process, that is, its continuity, is owing to this change of place between hydrogen and ethyl, first taking place in one direction and then in the opposite; that is, that sulphuric acid becomes sulphovinic acid by taking up ethyl instead of an atom of hydrogen, and that it is then re-converted into sulphuric acid by resuming hydrogen instead of this ethyl, the first change forming water, the second ether.
By using successively two different alcohols, it was shown that the two steps of this decomposition can be separated and their reality proved. The process of etherification is thus effected by a succession of double decompositions, each of which considered individually is perfectly conformable to the law of definite proportions; but the alternation and continuous succession so clearly proved in them, is a fact unexplained by that law. A complete analogy between this process and the more familiar cases of chemical action is therefore only to be established by finding in these latter a similar atomic motion.
A little reflection is sufficient to show that such a motion actually exists. The fact of diffusion is in reality nothing but a change of place between atoms, effected by the mere action of the particles of one another... .
 Back to the list of selected historical papers.
Back to the list of selected historical papers. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.