Determining the Formula of a Hydrate
Objectives
- Introduction to quantitative analysis: determination of proportions of subunits in a chemical sample
- Laboratory tool: Bunsen burner
- Introduction to forms of matter with example of ionic hydrates
Background: Forms of Matter
- Elements: Matter composed of only one type of atom (e.g. Na, He, O2, P4)
- Compounds: Matter composed of more than one type of atom. E.g.
- Na2SO4 (ionic compound; Na2SO4 is the formula unit)
- H2O (covalent; exists as separate H2O molecules)
- Molecules: Matter composed of more than one atom; the atoms are joined by covalent bonds (e.g., O2, P4, C6H12O6, TiCl4).
Ionic Hydrates
Ionic compounds (salts) in which one or more water molecules are bound in the crystalline structure of the salt:
CoCl2 | . | 6H2O
|
(anhydrous salt) | | (waters of hydration) |
| BaCl2.2H2O
| CaCO3.2 1/2 H2O
| CaSO4.5H2O |
There is a unique ratio of water molecules to formula units in the crystalline structure.
Interactions in an Ionic Hydrate
| BaCl2.3H2O | 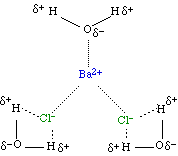 |
Procedure (Overview)
Procedure (Details)
Each student will work individually.
- Thoroughly wipe a crucible and cover with a clean cloth towel to remove dirt and other particulate matter.
Then determine the mass of the crucible (and cover) to +/- 0.01 g.
- Obtain about 1 g of a hydrate sample and transfer the sample to the crucible.
- Samples will be found on the reagent counter; be sure to write down the identification code.
- Test tubes containing 1 g of hydrate samples will also be found on the reagent counter. To quickly obtain about 1 g of hydrate sample, take a test tube from your locker and obtain about the same quantity of hydrate, from a reagent bottle, as you find in one of the reference samples. After you obtain your sample, quickly replce the cap to the reagent bottle and tighten securely.
- Transfer the hydrate sample to your crucible.
- Determine the mass of the crucible, hydrate sample, and crucible cover.
- Using your crucible tongs, place the crucible and contents back on the clay triangle. Partially cover the opening of the crucible with the cover.
- Heat your crucible and its contents with a low flame for 5 min. Increase the flame temperature and heat with a medium flame for 5 min. Further increase the flame and heat the sample for an additional 10 min. Do not allow the crucible to turn red. (Overheating may lead to decomposition of your sample!)
- Using crucible tongs, remove the crucible (with cover in place) from the clay triangle and place on wire gauze on the lab bench.
- Allow the crucible to cool to room temperature. (Hold your hand about 1 cm above the crucible to test.) Then determine the mass of the crucible and contents (and cover) to +/- 0.01 g.
- Heat your sample to constant mass.
- Reheat your crucible, with the cover ajar as before, for 10 min. using a hot flame. Allow the crucible to cool to room temperature and reweigh.
- The difference between the masses of the crucible plus sample, after the first and second heating, should be no greater than 0.03 g.
- If the difference is greater than 0.03 g, repeat the heating and cooling procedure until the difference between consecutive heatings is less than this limit.
- You will then have heated your sample to a "constant mass".
- After you have heated your crucible and contents to constant mass, transfer the crucible's contents to the disposal container.
- You may wish, if time permits, to perform a second determination.
- Determine the formula of the hydrate
- Samples 1, 3, and 5 are hydrates of magnesium sulfate, MgSO4.xH2O
Samples 2 and 4 are hydrates of zinc sulfate, ZnSO4.xH2O
- To determine the formula, you must determine the following
| # mols H2O | | # mols H2O
|
| x = | ______________ | ; or y = | ______________
|
| # mols MgSO4 | | # mols ZnSO4 |
- To determine the number of moles of anhydrous salt and of H2O:
| | | 1 mol MgSO4
|
| # mols MgSO4 = | g MgSO4 | x | ______________
|
| (measured mass of | | g MgSO4
|
| anhydrous salt) | | (1/molar mass)
|
|
| | | 1 mol H2O
|
| # mols H2O = | g H2O | x | ______________
|
| (mass of | | g H2O
|
| water lost) | | (1/molar mass)
|
 Back to the Chemical Principles Lab Schedule.
Back to the Chemical Principles Lab Schedule.
 Back to the Chemical Principles Lab Schedule.
Back to the Chemical Principles Lab Schedule.