Calorimetry of Acid-Base Neutralization
Objectives
- Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction vessel.
- Measurement of the enthalpy of neutralization (the heat evolved in an acid-base reaction) of a strong acid with a strong base. It will be necessary to measure the calorimeter constant of the calorimeter before we can do this.
Thermochemistry
- Qualitative
Many chemical reactions evolve (generate) heat, and some absorb heat. Reactions which evolve heat transfer that heat to their surroundings, causing the surroundings to become warmer; such reactions are called exothermic (meaning heat goes out). Reactions which absorb heat take heat from their surroundings, causing the surroundings to become cooler; such reactions are called endothermic (meaning heat goes in). The first page of the lab handout gives several examples of exothermic reactions and one endothermic reaction. Combustion reactions are strongly exothermic; that is why they are so widely used as commercial sources of heat.
- Quantitative
The quantity of heat generated or absorbed in a chemical reaction is the difference in heat content between products and reactants. The technical name for the heat content of a substance (at constant pressure) is the enthalpy, H. The amount of heat involved in a chemical reaction is the change in enthalpy, ΔH, defined as:
ΔH = H of products - H of reactants .
If the products contain more heat than the reactants, they must have absorbed heat from the surroundings; so if ΔH > 0, then ΔH is the amount of heat absorbed by an endothermic reaction. If the products contain less heat than the reactants, some heat must have been released into the surroundings; so if ΔH < 0, then |ΔH| (the absolute value of ΔH) is the amount of heat evolved by an exothermic reaction.
Calorimetry
The amount of heat absorbed or evolved by a chemical reaction can be determined by measuring the change in temperature in the surroundings, for that heat raises or lowers the temperature of the surroundings. (Note: Heat and temperature are certainly related, but they are not identical. Do not use the terms interchangeably. Calorimetry obtains information about flows of heat by measuring changes in temperature.)
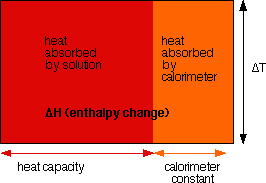
If the reaction takes place in a well-insulated container, than practically all the enthalpy change of the reaction will be confined to the container, raising or lowering the temperature of its contents. This is the basis of calorimetry. An insulated reaction container (which is called a calorimeter) is filled with a carefully measured quantity of a solution whose heat capacity is known. The heat capacity is the amount of heat needed to raise the solution by one degree. If we measure the temperature change of the contents of the calorimeter, then we can compute the amount of heat necessary to cause that temperature change.
In practice, the calorimeter is not perfectly insulated, so some heat escapes. We account for that escaping heat by measuring the temperature change during a process whose heat flows are known. By comparing the temperature change and the heat capacity to the known heat flow, we can determine how much heat escapes, and account for it. The amount of escaping heat per °C temperature change is called the calorimeter constant.
Procedure
- Work in pairs
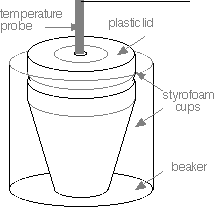
- Your calorimeter will consist of two nested styrofoam coffee cups and a plastic cover. Rather than thermometers, you will use a temperature sensor equipped with a thermocouple probe. There will be a hole in the plastic cover of your calorimeter for insertion of the probe. Place the nested coffee cups in a in a 250 mL beaker to lessen the probability of spillage.
Determining the Molar Enthalpy of Neutralization
You will use either HCl or H2SO4 as your acid (You may choose.), and NaOH as your base. The neutralization reactions are:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
H2SO4 (aq) + 2 NaOH (aq) → Na2SO4 (aq) + 2 H2O (l)
- Obtain four styrofoam cups and two plastic covers. Two of these cups will be used to construct the calorimeter where the mixing and reaction processes will occur. The other two cups and other cover will form a "duplicate calorimeter" to be used as an insulated container for one of the reagents. Be sure the cups are thoroughly dry before proceeding. Assemble two calorimeters; each is a nested pair of cups with a lid.
- Using a buret, add 50.0 mL of the acid to one calorimeter and put the plastic cover in place. Record the volume of acid.
- Using a second buret add 50.0 mL NaOH to the duplicate calorimeter. Put the plastic cover in place. Record the volume of base.
- Measure the temperature of the acid and base at 30-second intervals as specified on Data Sheet 1.
- Five minutes after the first measurement of the acid solution, pour the NaOH into the acid solution (in the calorimeter) rapidly and completely. Replace the cover, insert the temperature probe and, while stirring the contents of the calorimeter, take temperature measurements once every minute from minutes 6 through 13.
- Discard reaction mixture in the laboratory sinks and of Kimwipes in the trash.
- Plot temperature-time data and determine ΔT. (See calculations below.)
- Carry out a second determination with new portions of the solutions. Be sure to dry the calorimeters thoroughly before repeating.
Determining the Calorimeter Constant
The purpose of the following set of measurements is to determine a correction factor to be applied to the main measurements. The following measurements involve not a reaction, but the mixing of equal portions of warm and "cold" water in a temperature range similar to that of the neutralization reaction. If the calorimeter were perfectly insulated, the temperature after mixing would be exactly halfway between the temperatures of the hot and cold water; measurements on this system will get at how much heat is absorbed by the calorimeter.
- Dry the calorimeters thoroughly before proceeding. Measure out 50.0 mL of distilled water into the "reaction" calorimeter, and put a cover in place. This is the "cold" water sample.
- Measure another 50.0 mL of distilled water into a clean, dry beaker and heat, with occasional stirring, until the water is approximately 15°C above room temperature. Do not overheat the water: we want the temperature to be similar that of the reaction mixture. Pour the warm water into the duplicate calorimeter and put the cover in place. This is the "hot" water sample.
- Measure the temperature of the "cold" and "hot" water samples as specified on Data Sheet 2.
- Five minutes after the first measurement of the "cold" water, pour the sample of "hot" water into the calorimeter rapidly and completely. Replace the cover, insert the temperature probe and, while stirring the contents of the calorimeter, take temperature measurements once every minute from minutes 6 through 13.
- Plot the temperature-time data and calculate the calorimeter constant. (See calculations below.) Check with the instructor to see whether a second determination is necessary.
- If a second determination is necessary, be sure to dry the apparatus thoroughly before proceeding.
Calculations
ΔH = -CtotalΔT
where the heat capacity Ctotal = Cmixture + Ccalorimeter and ΔT is obtained from the graphs you will make of time-temperature data. The reason you take temperature readings over a period of time is so that you can draw the best straight lines through your data and extrapolate those lines to the time of mixing. That way you can separate the temperature change due to mixing (or reaction) from the temperature change due to heat entering or escaping the calorimeter from the outside.
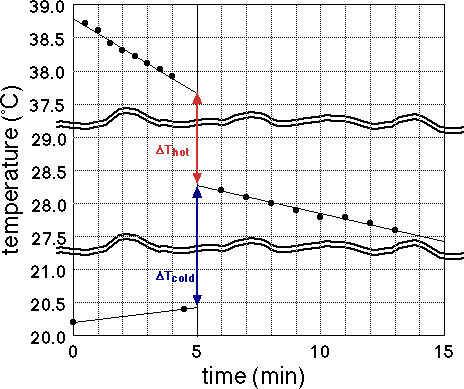
- Don't connect the dots!
- Extrapolate to line of mixing: Tinitial does not mean T at time zero, but rather T before mixing extrapolated to time of mixing. Similarly Tfinal does not mean the last temperature recorded, but rather T after mixing extrapolated back to the time of mixing.
- Make a break in the vertical scale if there is a large temperature range with no data points.
- Make your graph fill most of the page.
- Give your graphs appropriate titles that tells your instructor which part of the experiment he or she is looking at (such as "calibration run" or "mixing hot and cold water" for the mixing run and "neutralization run" for the mixing of acid and base).
 Back to the Chemical Principles Lab Schedule.
Back to the Chemical Principles Lab Schedule.

 Back to the Chemical Principles Lab Schedule.
Back to the Chemical Principles Lab Schedule.