Ernest Rutherford (1871-1937) and T. Royds
The Nature of the α Particle from Radioactive Substances.
E. Rutherford and T. Royds[1], Phil. Mag. 17, 281-6 (1909) [from Stephen Wright, ed., Classical Scientific Papers--Physics (New York: American Elsevier, 1964)]
The experimental evidence collected during the last few years has strongly supported the view that the α particle is a charged helium atom, but it has been found exceedingly difficult to give a decisive proof of the relation. In recent papers, Rutherford and Geiger[2] have supplied still further evidence of the correctness of this point of view. The number of α particles from one gram of radium have been counted, and the charge carried by each determined. The values of several radioactive quantities, calculated on the assumption that the α particle is a helium atom carrying two unit charges, have been shown to be in good agreement with the experimental numbers. In particular, the good agreement between the calculated rate of production of helium by radium and the rate experimentally determined by Sir James Dewar[3], is strong evidence in favour of the identity of the α particle with the helium atom.
The methods of attack on this problem have been largely indirect, involving considerations of the charge carried by the helium atom and the value of e/m of the α particle. The proof of the identity of the α particle with the helium atom is incomplete until it can be shown that the α particles, accumulated quite independently of the matter from which they are expelled, consist of helium. For example, it might be argued that the appearance of helium in the radium emanation was a result of the expulsion of the α particle, in the same way that the appearance of radium A is a consequence of the expulsion of an α particle from the emanation. If one atom of helium appeared for each α particle expelled, calculation and experiment might still agree, and yet the α particle itself might be an atom of hydrogen or of some other substance.
We have recently made experiments to test whether helium appears in a vessel into which the α particles have been fired, the active matter itself being enclosed in a vessel sufficiently thin to allow the α particles to escape, but impervious to the passage of helium or other radioactive products.
The experimental arrangement is clearly seen in the figure.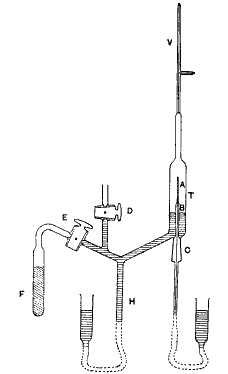 The equilibrium quantity of emanation from about 140 milligrams of radium was purified and compressed by means of a mercury-column into a fine glass tube A about 1.5 cms. long. This fine tube, which was sealed on a larger capillary tube B, was sufficiently thin to allow the α particles from the emanation and its products to escape, but sufficiently strong to withstand atmospheric pressure. After some trials, Mr. Baumbach succeeded in blowing such fine tubes very uniform in thickness. The thickness of the wall of the tube employed in most of the experiments was less than 1/100 mm., and was equivalent in stopping power of the α particle to about 2 cms. of air. Since the ranges of the α particles from the emanation and its products radium A and radium C are 4.3, 4.8, and 7 cms. respectively, it is seen that the great majority[4] of the α particles expelled by the active matter escape through the walls of the tube. The ranges of the α particles after passing through the glass were determined with the aid of a zinc-sulphide screen. Immediately after the introduction of the emanation the phosphorescence showed brilliantly when the screen was close to the tube, but practically disappeared at a distance of 5 cms. Such a result is to be expected. The phosphorescence initially observed was due mainly to the α particles of the emanation and its product radium A (period 3 mins.). In the course of time the amount of radium C, initially zero, gradually increased, and the α radiations from it of range 7 cms. were able to cause phosphorescence at a greater distance.
The equilibrium quantity of emanation from about 140 milligrams of radium was purified and compressed by means of a mercury-column into a fine glass tube A about 1.5 cms. long. This fine tube, which was sealed on a larger capillary tube B, was sufficiently thin to allow the α particles from the emanation and its products to escape, but sufficiently strong to withstand atmospheric pressure. After some trials, Mr. Baumbach succeeded in blowing such fine tubes very uniform in thickness. The thickness of the wall of the tube employed in most of the experiments was less than 1/100 mm., and was equivalent in stopping power of the α particle to about 2 cms. of air. Since the ranges of the α particles from the emanation and its products radium A and radium C are 4.3, 4.8, and 7 cms. respectively, it is seen that the great majority[4] of the α particles expelled by the active matter escape through the walls of the tube. The ranges of the α particles after passing through the glass were determined with the aid of a zinc-sulphide screen. Immediately after the introduction of the emanation the phosphorescence showed brilliantly when the screen was close to the tube, but practically disappeared at a distance of 5 cms. Such a result is to be expected. The phosphorescence initially observed was due mainly to the α particles of the emanation and its product radium A (period 3 mins.). In the course of time the amount of radium C, initially zero, gradually increased, and the α radiations from it of range 7 cms. were able to cause phosphorescence at a greater distance.
The glass tube A was surrounded by a cylindrical glass tube T, 7.5 cms. long and 1.5 cms. diameter, by means of a ground-glass joint C. A small vacuum-tube V was attached to the upper end of T. The outer glass tube T was exhausted by a pump through the stopcock D, and the exhaustion completed with the aid of the charcoal tube F cooled by liquid air. By means of a mercury column H attached to a reservoir, mercury was forced into the tube T until it reached the bottom of the tube A.
Part of the α particles which escaped through the walls of the fine tube were stopped by the outer glass tube and part by the mercury surface. If the α particle is a helium atom, helium should gradually diffuse from the glass and mercury into the exhausted space, and its presence could then be detected spectroscopically by raising the mercury and compressing the gases into the vacuum-tube.
In order to avoid any possible contamination of the apparatus with helium, freshly distilled mercury and entirely new glass apparatus were used. Before introducing the emanation into A, the absence of helium was confirmed experimentally. At intervals after the introduction of the emanation the mercury was raised, and the gases in the outer tube spectroscopically examined. After 24 hours no trace of the helium yellow line was seen; after 2 days the helium yellow was faintly visible; after 4 days the helium yellow and green lines were bright; and after 6 days all the stronger lines of the helium spectrum were observed. The absence of the neon spectrum shows that the helium present was not due to a leakage of air into the apparatus.
There is, however, one possible source of error in this experiment. The helium may not be due to the α particles themselves, but may have diffused from the emanation through the thin walls of the glass tube. In order to test this point the emanation was completely pumped out of A, and after some hours a quantity of helium, about 10 times the previous volume of the emanation, was compressed into the same tube A.
The outer tube T and the vacuum-tube were removed and a fresh apparatus substituted. Observations to detect helium in the tube T were made at intervals, in the same way as before, but no trace of the helium spectrum was observed over a period of eight days.
The helium in the tube A was then pumped out and a fresh supply of emanation substituted. Results similar to the first experiment were observed. The helium yellow and green lines showed brightly after four days.
These experiments thus show conclusively that the helium could not have diffused through the glass walls, but must have been derived from the α particles which were fired through them. In other words, the experiments give a decisive proof that the α particle after losing its charge is an atom of helium.
Other Experiments.
We have seen that in the experiments above described helium was not observed in the outer tube in sufficient quantity to show the characteristic yellow line until two days had elapsed. Now the equilibrium amount of emanation from 100 milligrams of radium should produce helium at the rate of about .03 c.mm. per day. The amount produced in one day, if present in the outer tube, should produce a bright spectrum of helium under the experimental conditions. It thus appeared probable that the helium fired into the glass must escape very slowly into the exhausted space, for if the helium escaped at once, the presence of helium should have detected a few hours after the introduction of the emanation.
In order to examine this point more closely the experiments were repeated, with the addition that a cylinder of thin sheet lead of sufficient thickness to stop the α particles was placed over the fine emanation tube. Preliminary experiments, in the manner described later, showed that the lead-foil did not initially contain a detectable amount of helium. Twenty-four hours after the introduction into the tube A of about the same amount of emanation as before, the yellow and green lines of helium in this case after one day was of about the same intensity as that after the fourth day in the experiments without the lead screen. It was thus clear that the lead-foil gave up the helium fired into it far more readily than the glass.
In order to form an idea of the rapidity of escape of the helium from the lead some further experiments were made. The outer cylinder T was removed and a small cylinder of lead-foil placed round the thin emanation-tube surrounded the air at atmospheric pressure. After exposure for a definite time to the emanation, the lead screen was removed and gested [sic--tested?] for helium as follows. The lead-foil was placed in a glass tube between two stopcocks. In order to avoid a possible release of the helium present in the lead by pumping out the air, the air was displaced by a current of pure electrolytic oxygen[5]. The stopcocks were closed and the tube attached to a subsidiary apparatus similar to that employed for testing for the presence of neon and helium in the gases produced by the action of the radium emanation on water (Phil. Mag. Nov. 1908). The oxygen was absorbed by charcoal and the tube then heated beyond the melting-point of lead to allow the helium to escape. The presence of helium was then spectroscopically looked for in the usual way. Using this method, it was found possible to detect the presence of helium in the lead which had been exposed for only four hours to the α rays from the emanation. After an exposure of 24 hours the helium yellow and green lines came out brightly. These experiments were repeated several times with similar results.
A number of blank experiments were made, using samples of the lead-foil which had not been exposed to the α rays, but in no case was any helium detected. In a similar way, the presence of helium was detected in a cylinder of tinfoil exposed for a few hours over the emanation-tube.
These experiments show that the helium does not escape at once from the lead, but there is on the average a period of retardation of several hours and possible longer.
The detection of helium in the lead and tin foil, as well as in the glass, removes a possible objection that the helium might have been in some way present in the glass initially, and was liberated as a consequence of its bombardment by the α particles.
The use of such thin glass tubes containing emanation affords a simple and convenient method of examining the effect on substances of an intense α radiation quite independently of the radioactive material contained in the tube.
We can conclude with certainty from these experiments that the α particle after losing its charge is a helium atom. Other evidence indicates that the charge is twice the unit charge carried by the hydrogen atom set free in the electrolysis of water.
University of Manchester,
Nov. 13, 1908
[1] Communicated by the Authors.
[2] Proc. Roy. Soc. A. lxxxi, pp. 141-173 (1908).
[3] Proc. Roy. Soc. A. lxxxi. p. 280 (1908).
[4] The α particles fired at a very oblique angle to the tube would be stopped in the glass. The fraction stopped in this way would be small under the experimental conditions.
[5] That the air was completely displaced was shown by the absence of neon in the final spectrum.
 Back to the list of selected historical papers.
Back to the list of selected historical papers.
 Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.
 The equilibrium quantity of emanation from about 140 milligrams of radium was purified and compressed by means of a mercury-column into a fine glass tube A about 1.5 cms. long. This fine tube, which was sealed on a larger capillary tube B, was sufficiently thin to allow the α particles from the emanation and its products to escape, but sufficiently strong to withstand atmospheric pressure. After some trials, Mr. Baumbach succeeded in blowing such fine tubes very uniform in thickness. The thickness of the wall of the tube employed in most of the experiments was less than 1/100 mm., and was equivalent in stopping power of the α particle to about 2 cms. of air. Since the ranges of the α particles from the emanation and its products radium A and radium C are 4.3, 4.8, and 7 cms. respectively, it is seen that the great majority[4] of the α particles expelled by the active matter escape through the walls of the tube. The ranges of the α particles after passing through the glass were determined with the aid of a zinc-sulphide screen. Immediately after the introduction of the emanation the phosphorescence showed brilliantly when the screen was close to the tube, but practically disappeared at a distance of 5 cms. Such a result is to be expected. The phosphorescence initially observed was due mainly to the α particles of the emanation and its product radium A (period 3 mins.). In the course of time the amount of radium C, initially zero, gradually increased, and the α radiations from it of range 7 cms. were able to cause phosphorescence at a greater distance.
The equilibrium quantity of emanation from about 140 milligrams of radium was purified and compressed by means of a mercury-column into a fine glass tube A about 1.5 cms. long. This fine tube, which was sealed on a larger capillary tube B, was sufficiently thin to allow the α particles from the emanation and its products to escape, but sufficiently strong to withstand atmospheric pressure. After some trials, Mr. Baumbach succeeded in blowing such fine tubes very uniform in thickness. The thickness of the wall of the tube employed in most of the experiments was less than 1/100 mm., and was equivalent in stopping power of the α particle to about 2 cms. of air. Since the ranges of the α particles from the emanation and its products radium A and radium C are 4.3, 4.8, and 7 cms. respectively, it is seen that the great majority[4] of the α particles expelled by the active matter escape through the walls of the tube. The ranges of the α particles after passing through the glass were determined with the aid of a zinc-sulphide screen. Immediately after the introduction of the emanation the phosphorescence showed brilliantly when the screen was close to the tube, but practically disappeared at a distance of 5 cms. Such a result is to be expected. The phosphorescence initially observed was due mainly to the α particles of the emanation and its product radium A (period 3 mins.). In the course of time the amount of radium C, initially zero, gradually increased, and the α radiations from it of range 7 cms. were able to cause phosphorescence at a greater distance. Back to the list of selected historical papers.
Back to the list of selected historical papers.