In the paper in which I proved that the two radio-elements, mesothorium I, and radium, are non-separable by chemical processes, and by fractional crystallisation of the chlorides, although the atomic weight of the two elements differs by about two units, it was pointed out that some of the common elements might also be mixtures of non-separable elements of different atomic weight in constant proportions. In a recent book ("Chemistry of the Radio-elements," p. 30) I stated the rule that held good in several cases, that when the α-particle was expelled the atom passed from a family of even number in the Periodic Table to the next lower-numbered even family, the family of odd number being always missed. Further, in the changes in which the α-particle was not expelled the atom in several cases reverted to its original group, resulting in a curious alternation of properties as the series proceeds. Now, when this occurs, an element of the fourth family, for example, expelling an α-particle and becoming a member of the second family, which after further changes reverts to the fourth family, the two representatives of the fourth family so resulting are not merely similar in chemical properties. They are non-separable by any known process. This applies not merely to the disintegration products of one series but to all the products. Thus, in the fourth group, thorium, uranium X, ionium, radio-thorium, radio-actinium are all chemically non-separable, though they may result from three separate series, and the calculated atomic weight varies from 234 to 228.
I suggested to my demonstrator, Mr. Alexander Fleck, that he should make a systematic investigation of as many of the radio-elements as possible, the chemical nature of which remained indefinite, and the first part of his results has recently been communicated to the Chemical Society (Proc. Chem. Soc., 1913, xxix., 7). As the result of this work at the present time it is possible to state or predict the chemical nature of every known member of the disintegration series, and to bring these series from end to end under a few general laws of the type described, which throw a flood of new light on the nature of the Periodic Law. The lacunae that still remain to be filled up between uranium and ionium, owing to existence in this part of products with periods of the order of millions of years, can be discussed in a much narrower way in consequence.
In a paper published by A. S. Russell recently (Chemical News, 1913, cvii., 49) some of these generalisations have already been dealt with. Mr. Russell put forward a corollary to my rule for the α-particle which he had previously communicated to me privately in a letter in October, 1912, and which has since been strikingly verified by some of Mr. Fleck's results. Mr. Russell's rule refers to the β-ray and rayless changes, and is that when a β-ray or rayless change occurs the atom changes in chemical nature so as to pass into the family in the Periodic Table next higher in number. That is, the passage in these cases is always from an even to an odd, or from an odd to an even-numbered family. G. von Hevesy (Phys. Zeit., 1913, xiv., 49) who has also been working in Prof. Rutherford's laboratory on the valency of the disintegration products, has put forward very similar views, the difference being that the effect of the β-ray change is considered by him to be the opposite or "polar" to that of the α-ray change, the valency increasing by two after a β-ray change.
The same questions are also very clearly discussed by K. Fajans, who has been connected with the development of our knowledge in the branch series, but his paper did not come to hand until after this paper was drafted (Phys. Zeit., 1913, xiv., 131 and 136). He takes the view here advocated that the Periodic Law is the expression of the periodic character of radio-active changes, and anticipates some of the other points dealt with in this paper.
There is no doubt that Mr. Russell's corollary to my α-ray rule is correct, and I have adopted it just as he has put it forward, but it is possible from Mr. Fleck's results still to learn a good deal that is quite definite as to the nature of radio-active change.
In the first place let us consider the radium series from the emanation to the ultimate product with the branch series that occurs at the C-member. These branch series are now fairly clear owing to the work of Barratt, Marsden, and Darwin in the thorium series, and Makower and Fajans in the radium series, and as I have discussed them fully from the standpoint of the theory of multiple disintegration in the "Annual Report on Radio-activity for 1912" (Ann. Reports of Progress of Chemistry, Chemical Society, 1912), I need not further deal with them here.
Apart from the definite recognition of polonium (RaF) as the homologue of tellurium, and of radium D as non-separable from lead, there was practically nothing known of the chemistry of the other members. From von Lerch's rule and the easy deposition on metals before the phenomenon had been recently investigated from the electrochemical standpoint by v. Hevesy (Phil. Mag., 1912, [6], xxiii., 628) the impression had become general that they might be allied to the noble metals.
From Fleck's results the series runs:-- A, unknown; B, lead; C, bismuth; C', unknown; D, lead, E, bismuth; F, polonium; G, lead. The known members are in accord with the rule about the expulsion of α- and β-particles, and if we extend the rule to the members the chemistry of which is still unknown, it gives for the members of the families of the successive members:-- A, VI.; B, IV.; C, V.; C', VI.; D, IV.; E, V.; F, VI.; G, IV. Now, applying the rule that when similar groups recur the elements are not merely similar, but non-separable, we can predict that in chemical behaviour the two unplaced substances RaA and RaC' will be non-separable from polonium. The prediction with regard to C' is unverifiable, as the period of this body is estimated to be only 10-6 sec. But with regard to RaA it should be possible to test it, and this is now being done. As regards the branch series, which is difficult to investigate, as only some three out of 10,000 of the atoms choose this route, it may be predicted that RaC2 is in the third family, and will prove to be non-separable from thallium, as will later be discussed. The end-product of the branch is again lead. The atomic weight of the two end-products, both non-separable from lead, are about 206 and 210 respectively.
In discussing the thorium series similarly, it must be noted that the product termed thorium D is not the analogue of radium D, but of radium C2, as thorium D is formed from thorium C', the product giving the longest α-rays, and therefore possessing the shortest life known (estimated at 10-11 second).
It is hardly necessary to discuss it in detail. Thorium A and C' should be non-separable from polonium, though their periods are too short for this to be determinable. Both end-products, formed in this case in the proportion 65 to 35, should be non-separable from lead, the calculated atomic weight of this "lead" being about 208.5 in each case. ThD, like RaC2, would prove to be non-separable from thallium.
The actinium series from the emanation is precisely analogous, except that no branch series has yet been established. Probably one will be found to exist, but as in radium it may represent an insignificant proportion only of the atoms disintegrating. If actinium is assumed to be analogous to thorium, again both products are non-separable from lead. The main series is analogous to that of thorium and the opposite of that of radium, actinium D being formed in the α-ray change of actinium C.
The series before the emanations furnish also, as Mr. Russell has shown, perfect illustration of the applicability of the rules with regard to α- and β-changes.
In the thorium series Fleck has shown that mesothorium II., the only member the chemistry of which remained unknown, is non separable from actinium. Mr. Cranston in this laboratory is just completing a research, in which he has proved that radio-thorium is the direct product of mesothorium II., a lacuna that had previously not been filled up, and which is important because the parent of ionium, the product in the uranium series corresponding with radio-thorium in the thorium series, is still experimentally unknown. The actinium series is almost absolutely analogous with the thorium series, actinium itself corresponding with mesothorium II., from which it is non-separable, the only difference being that actinium is rayless like mesothorium I., instead of giving β- and γ-rays like mesothorium II. Fleck has shown that radio-actinium, the chemistry of which had remained indefinite, is non-separable from thorium. Russell and Chadwick have described the separation of a body giving α- and β- and γ-rays from radio-actinium, but as radio-actinium is not known to give γ-rays and only gives very soft β-rays, it seems advisable to await further results.
The radium series from ionium onwards is precisely similar to that of thorium from radio-thorium onwards, but lacunae remain in the connection between ionium and uranium. This part of the table I have been engaged in examining for the last ten years, and my results, though negative for the most part, at least exclude many possibilities. It is clear, as Mr. Russell and several others have pointed out, that uranium X must be the product of uranium I. and not of Uranium II., as hitherto generally assumed. Because the product of uranium I., an α-particle being expelled must belong to the fourth group, and for this to get back to the sixth group at uranium II., two β or rayless changes must ensue. Similarly, the unknown product of uranium X must belong to the fifth group, nowhere else represented, and be the homologue of tantalum. Calling this ekatantalum [eka Ta] it is possible to predict a good deal about its properties from general principles, and the results of my experiments on the product of uranium X (Phil. Mag., 1909, [6], xviii., 858; 1910, xx., 342). It can only give α rays if its period is extraordinarily long. If it does not give α-rays its product must be in the sixth group. So that with fair probability we may write, as Russell does:--
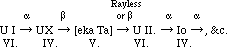
Now, the α-ray producing members all belong to the even families, except in the case of the members where dual disintegration occurs. The C-members, like [eka Ta], belong to the fifth family, and undergo a dual change, such that in one branch a β-ray is followed by an α-ray change, and in the other an α-ray change is followed by a β-ray change. It is scarcely possible to resist the temptation of supposing that such a change is actually going on in [eka Ta], the rayless change into U II., followed by the α-ray change of that substance, in the one branch, proceeding simultaneously with an α-ray change into actinium, which, as is known, changes raylessly, in the other branch.
With regard to the experiments on the products of uranium X in which no growth of α-rays could be detected during the decay of the β-rays, it may be pointed out that a very small growth would be masked by the simultaneous decay of the undeviable β-rays at the same rate. Further, all the products gave α-rays after the β-radiation had disappeared. The first products possessed an activity, which consisted largely of actinium, but in the last products separated, this constituent could only just be detected initially by the active deposit test.
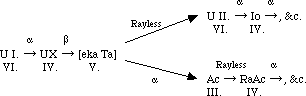
I have some very slight evidence that actinium is really produced from uranium X through an intermediate substance. The actinium present in the last two lots of uranium X preparations, separated from 50 kilogrms. of uranium in June and September, 1909, has been accurately measured over a term of four years, and the measurements reveal an excessively minute but regular growth. In the table the activity of the active deposit produced from the preparations at different times is shown.
| Days from preparation. | ||||
| 328. | 432. | 829. | 1282. | |
| Activity (divisions per minute) | ||||
| due to actinium active | ||||
| deposit | 1.43 (?) | 1.53 | 1.70 | 1.90 |
The effects are excessively feeble. The conditions of the first measurement were not absolutely comparable with the others, and this is the meaning of the (?). It is clear that much further time must elapse before such measurements can be depended upon for proof of the growth of actinium. If the above scheme is correct it is certain that the period of [eka Ta] must be very long, but as no information exists as to the period of actinium it is impossible to give an estimate. In the meantime the very definite view it is now possible to take of the nature of [eka Ta] should facilitate the search for this missing element, and lead to the problem being more directly elucidated.
Without a single exception, though some cases still remain to be studied, the rule is borne out that all members in all three disintegration series placed in the same family are non-separable from one another. When any place in the Periodic Table was before unoccupied the radio-element first discovered to be occupying it appeared as a new type. Thus the homologues of barium and tellurium respectively were unknown before radio-active investigations. Radium and polonium appeared as new types separable from barium and tellurium respectively. But the products radio-thorium, uranium X, ionium, and radio-actinium in the fourth group occupy a place already occupied by the known element thorium, and hence are non-separable from thorium.
This enables it to be predicted that eka-tantalum will be separable from tantalum. Actinium also will prove to be separable from lanthanum, as Geisel has stated, but not yet clearly demonstrated.
When the zero group is crossed the place in the sixth family, before occupied by uranium, is now occupied by polonium, in the fifth by bismuth, in the fourth by lead, in the third by thallium. That is, after passing the zero group an entirely fresh set of types is met with, which is shown in the ordinary periodic arrangement by dividing the families into A and B sub-groups. We may predict that the A members and the C' members of the active deposits will be non-separable from polonium, and that RaC2 and ThD, possibly AcD, will be not merely analogous to thallium but non-separable from it.
These results prove that almost every vacant place in the Periodic Table between thallium and uranium is crowded with non-separable elements of atomic weight varying over several units, and leads inevitably to the presumption that the same may be true in other parts of the table. As previously pointed out, nothing further is necessary to explain the failure of all attempts to obtain numerical relations between the atomic weights. The view that the atomic mass is a real constant fixing all the chemical and physical properties of the elements is combatted most definitely by the fact that after the α-particle of mass 4 is expelled the members revert later to the original chemical type.
Finally, it may be predicted that all the end-products, probably six in number of the three series, with calculated atomic weights varying from 210 to 206, should be non-separable from lead; that is, should be "lead," the element that appears in the International List with the atomic weight 207.1. I should mention that Mr. Russell a year ago told me that he believed that the discrepancy between this value and that calculated (206.0) from the atomic weight of radium by the subtraction of five α-particles was due to the end-product of radium not being lead, but an element non-separable from it. It appears from the foregoing that all the end-products are probably non-separable from lead, and that "lead" is actually such a mixture as formerly, I supposed, might exist among the inactive elements.
If we suppose that all the lead in the world is produced as the end-products in the three disintegration series in constant proportion, the found atomic weight, 207.1, indicates that about half of it may result in that of thorium and the other half in that of uranium. It is, however, hardly profitable to go further in detail until the constancy of the atomic weight of lead from a variety of radio-active minerals has been experimentally tested.
A chart accompanies this paper, showing graphically the evolution of the elements through the Periodic Table from uranium to lead.
Physical Chemistry Laboratory,
Glasgow University, February 18, 1913.
Note added February 22, 1913.-- I should have mentioned that if the scheme given is correct and ionium is the direct product of the α-ray change of uranium II.--and it is hardly possible to frame any alternative agreeing with the rules of the α- and β-ray changes laid down--my estimate of the life of ionium as at least 100,000 years (Royal Institute Friday Evening Lecture, March 15th, 1912), becomes definite. It is dependent only upon the absence of long-lived substances, intermediate between these two elements. It follows, therefore, from the experiments of Exner and Hascheck, and A. S. Russell and Rossi, discussed fully in my "Annual Report on Radio-activity, 1913" (Section Ionium), in which these investigators failed to observe a single new line in the arc spectrum of ionium thorium preparations, that ionium and thorium must give the same arc spectrum. For on my minimum estimate of the life, the proportion of ionium in the preparations must have been at least 10 per cent by weight. The identity of the spectra of two non-separable elements, although at first sight a somewhat startling deduction, is in agreement with the modern view that the spectrum does not reveal the inner constitution of the atom, as previously assumed, but only its external characteristics. The electrons giving rise to spectra are probably those which condition valency and chemical properties, and are not what have been termed "constitutional electrons." Such "constitutional electrons" are, of course, merely a name for the material atom itself, considered apart from its atomic satellites.
I also take the opportunity of mentioning that Mr. Fleck tells me that he can effect the quantitative separation of thorium D, from the B and C members of the active deposit group, by precipitating potassium in the solution by means of platinic chloride. Taken in conjunction with the other reactions this is in conformity with the prediction that the D members of thorium and actinium will prove to be non-separable from thallium. --F.S.
 Back to the list of selected historical papers.
Back to the list of selected historical papers. Back to the top of Classic Chemistry.
Back to the top of Classic Chemistry.